Tetrahedrane on:
[Wikipedia]
[Google]
[Amazon]
Tetrahedrane is a hypothetical platonic hydrocarbon with
 The tetrahedrane motif occurs broadly in chemistry.
The tetrahedrane motif occurs broadly in chemistry. 
chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
and a tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phospho ...
.
C4 tetrahedranes
Tetrahedrane () is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane remains elusive, although predicted kinetically stable. One strategy that has been explored (but thus far failed) is reaction ofpropene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like od ...
with atomic carbon.
Contrariwise, several organic compounds with the tetrahedrane core are known. All have multiply bulky substituents, ''tert''-butyl (''t''-Bu) or larger. Locking a tetrahedrane molecule inside a fullerene has only been attempted ''in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on a computer or via computer simulation software. The phrase is pseudo-Latin for 'in silicon' (correct ), referring to silicon in computer chips. It was c ...
''.
All known syntheses have relied on rearrangement from another unstable moiety. In Maier's original synthesis, photochemical cheletropic decarbonylation converts a cyclopentadienone to the tetrahedrane. In a later synthesis, irradiation directly converted a cyclobutadiene to tetrahedrane. And more recently, single-electron oxidation can induce a radical chain isomerization with the same effect.
Tetrahedrane with small substituents would have a variety of interesting properties. Due to its bond strain and stoichiometry, tetranitrotetrahedrane has potential as a high-performance energetic material (explosive).
Calculations
A calculation is a deliberate mathematical process that transforms a plurality of inputs into a singular or plurality of outputs, known also as a result or results. The term is used in a variety of senses, from the very definite arithmetical ...
suggest that tetrahedrane's molecular strain reduces if slightly-flexible diyne spacers separate the vertices.
Tetra-''tert''-butyltetrahedrane
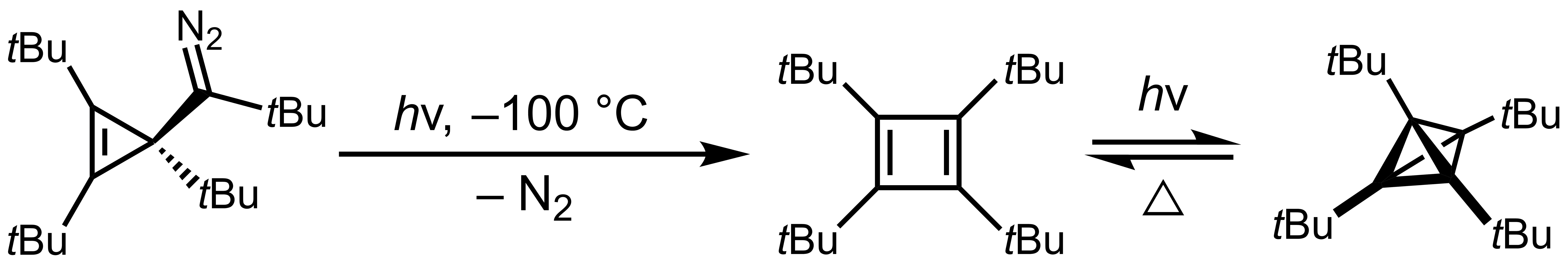
In 1978, Günther Maier first prepared tetra-''tert''-butyl-tetrahedrane, with a deceptively short and simple synthesis that required "astonishing persistence and experimental skill". "The relatively straightforward scheme shown ..conceals both the limited availability of the starting material and the enormous amount of work required in establishing the proper conditions for each step." In Maier's own account, it took several years of careful observation and optimization to develop the correct conditions for the reactions. For instance, the synthesis of tetrakis(''t-''butyl)cyclopentadienone from the tris(''t''-butyl)bromocyclopentadienone (itself synthesized with much difficulty) required over 50 attempts before working conditions could be found. Maier began with cycloaddition of analkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
to ''t''-Bu substituted maleic anhydride. Rearrangement and decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
gave a corset-stabilized cyclopentadienone. To add the fourth ''t''-Bu group, Maier brominated the only labile hydrogen to give an electrophile that coupled directly to tert-butyllithium
''tert''-Butyllithium is a chemical compound with the Chemical formula, formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base (chemistry), base, capable of deprotonation, deprotonating ...
. Photochemical cheletropic decarbonylation then gave the target.
:
Heating tetra-''tert''-butyltetrahedrane gives tetra-''tert''-butyl cyclobutadiene. The reversibility of this rearrangement proved key to developing a more scalable synthesis. In the last step, photolysis of a cyclopropenyl-substituted diazomethane affords the desired product through a tetrakis(''tert''-butyl)cyclobutadiene intermediate:
:
Trimethylsilyl tetrahedranes
Tetrakis(trimethylsilyl)tetrahedrane can be prepared by treatment of the cyclobutadiene precursor with tris(pentafluorophenyl)borane and is far more stable than the ''tert''-butyl analogue. The silicon–carbon bond is longer than a carbon–carbon bond, and therefore the corset effect is reduced. Whereas the ''tert''-butyl tetrahedrane melts at 135 °C concomitant with rearrangement to the cyclobutadiene, tetrakis(trimethylsilyl)tetrahedrane, which melts at 202 °C, is stable up to 300 °C, at which point it cracks to bis(trimethylsilyl)acetylene. The tetrahedrane skeleton is made up of banana bonds, and hence the carbon atoms are high in s-orbital character. FromNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
, sp- hybridization can be deduced, normally reserved for triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s. As a consequence the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s are unusually short with 152 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth o ...
s.
Reaction with methyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
with tetrakis(trimethylsilyl)tetrahedrane yields tetrahedranyllithium. The lithium compound can then couple to electrophiles, even relatively small ones.
A bis(tetrahedrane) has also been reported. The connecting bond is even shorter with 143.6 pm. An ordinary carbon–carbon bond has a length of 154 pm.
:
Tetrahedranes with non-carbon core atoms
White phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phospho ...
(P4) and yellow arsenic (As4) naturally form tetrahedrane-like clusters. There are a wide variety of synthetic pnictogen-substituted tetrahedranes, and metallatetrahedranes with a single metal (or phosphorus atom) capping a cyclopropyl trianion also exist. Several metal carbonyl clusters are referred to as tetrahedranes, e.g. tetrarhodium dodecacarbonyl.
:
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
also can be induced to form a tetrahedral core, but heavier adamantogens tend to form cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substanc ...
-like clusters.
Tetrasilatetrahedrane
In tetrasilatetrahedrane features a core of foursilicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
atoms. The standard silicon–silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group i ...
groups, which confer stability. The silatetrahedrane can be reduced with potassium graphite to the tetrasilatetrahedranide potassium derivative. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be sequestered by a crown ether, and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si−–Si bonds is now 272 pm and the tetravalent silicon atom of that bond has an inverted tetrahedral geometry. Furthermore, the four cage silicon atoms are equivalent on the NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
timescale due to migrations of the silyl substituents over the cage.
:
The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane. In this tetrahedrane the cage is protected by four so-called supersilyl groups in which a silicon atom has 3 ''tert''-butyl substituents. The dimer does not materialize but a reaction with iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
in benzene followed by reaction with the tri-''tert''-butylsilaanion results in the formation of an eight-membered silicon cluster compound
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semic ...
which can be described as a dumbbell (length 229 pm and with inversion of tetrahedral geometry) sandwiched between two almost-parallel rings.
:
See also
* Dodecahedrane * Prismane * PrismaneReferences
{{reflist, 30em Cycloalkanes Cluster chemistry Hypothetical chemical compounds Tricyclic compounds Tetrahedra