Stöber process on:
[Wikipedia]
[Google]
[Amazon]
The Stöber process is a chemical process used to prepare
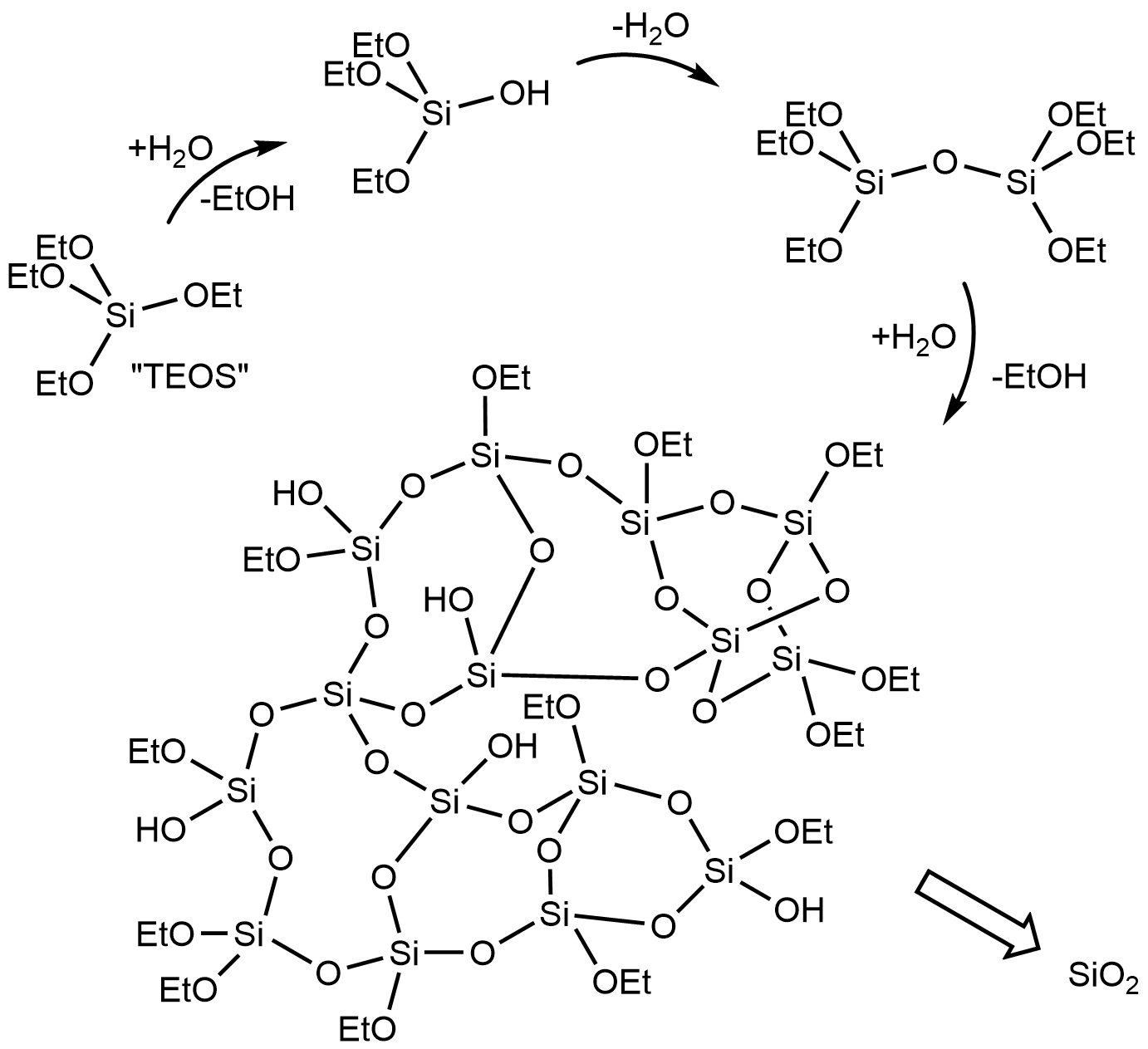 The Stöber process is a sol-gel approach to preparing monodisperse (uniform) spherical
The Stöber process is a sol-gel approach to preparing monodisperse (uniform) spherical Si(OEt)4 + H2O -> Si(OEt)3OH + EtOH
: Si(OEt)4 + 2H2O -> Si(OEt)2(OH)2 + 2EtOH
The reaction produces ethanol and a mixture of ethoxy2Si(OEt)3OH -> (EtO)3Si-O-Si(OEt)3 + H2O
: Si(OEt)3OH + Si(OEt)4 -> (EtO)3Si-O-Si(OEt)3 + EtOH
: Si(OEt)3OH + Si(OEt)2(OH)2 -> (EtO)3Si-O-Si(OEt)2OH + H2O
Further hydrolysis of the ethoxy groups and subsequent condensation leads to
silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
() particles of controllable and uniform size for applications in materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry
Wet chemistry is a form of analytical chemistry that uses classical methods such as observation to analyze materials. The term ''wet chemistry'' is used as most analytical work is done in the liquid phase. Wet chemistry is also known as ''bench c ...
synthetic approach to silica nanoparticles
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
. It is an example of a sol-gel process wherein a molecular precursor (typically tetraethylorthosilicate) is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism
Mechanism may refer to:
*Mechanism (economics), a set of rules for a game designed to achieve a certain outcome
**Mechanism design, the study of such mechanisms
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a ...
a particle aggregation
Particle agglomeration refers to the formation of assemblages in a suspension (chemistry), suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid ...
model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.
In 1999 a two-stage modification was reported that allowed the controlled formation of silica particles with small holes. The process is undertaken at low pH in the presence of a surface-active molecule. The hydrolysis step is completed with the formation of a microemulsion before adding sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes ...
to nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
the condensation process. The non-ionic surfactant is burned away to produce empty pores, increasing the surface area and altering the surface characteristics of the resulting particles, allowing for much greater control over the physical properties of the material. Development work has also been undertaken for larger pore structures such as macroporous monoliths, shell-core particles based on polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is an aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. It forms Coordination complex, coordination complexes with metal cations and is used to synthesize the chelating agent DOTA (chelator) ...
, or polyamines
A polyamine is an organic compound having two or more amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, Hygroscopy, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium de ...
, and carbon spheres.
Silica produced using the Stöber process is an ideal material to serve as a model for studying colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others exte ...
phenomena because of the monodispersity (uniformity) of its particle sizes. Nanoparticles prepared using the Stöber process have found applications including in the delivery of medications to within cellular structures and in the preparation of biosensor
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector.
The ''sensitive biological element'', e.g. tissue, microorganisms, organelles, cell rece ...
s. Porous silica Stöber materials have applications in catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
and liquid chromatography due to their high surface area
The surface area (symbol ''A'') of a solid object is a measure of the total area that the surface of the object occupies. The mathematical definition of surface area in the presence of curved surfaces is considerably more involved than the d ...
and their uniform, tunable, and highly ordered pore structures. Highly effective thermal insulator
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with s ...
s known as aerogel
Aerogels are a class of manufacturing, synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid wit ...
s can also be prepared using Stöber methods, and Stöber techniques have been applied to prepare non-silica aerogel systems. Applying supercritical drying
Supercritical drying, also known as critical point drying, is a process to remove liquid in a precise and controlled way. It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel ...
techniques, a Stöber silica aerogel with a specific surface area
Specific surface area (SSA) is a property of solids defined as the total surface area (SA) of a material per unit mass, (with units of m2/kg or m2/g). Alternatively, it may be defined as SA per solid or bulk volume (units of m2/m3 or m−1).
I ...
of 700 m2⋅g−1 and a density of 0.040 g⋅cm−3 can be prepared. NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
has prepared silica aerogels with a Stöber-process approach for both the Mars ''Pathfinder'' and '' Stardust'' missions.
One-step process
 The Stöber process is a sol-gel approach to preparing monodisperse (uniform) spherical
The Stöber process is a sol-gel approach to preparing monodisperse (uniform) spherical silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
() materials that was developed by a team led by Werner Stöber and reported in 1968. The process, an evolution and extension of research described in Gerhard Kolbe's 1956 PhD dissertation, was an innovative discovery that still has wide applications more than 50 years later. Silica precursor tetraethyl orthosilicate (, TEOS) is hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
in alcohol (typically methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
or ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
) in the presence of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
:
: silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon c ...
s (such as , , and even ), which can then condense
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
with either TEOS or another silanol with loss of alcohol or water:
: crosslink
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing. It is a one-step process as the hydrolysis and condensation reactions occur together in a single reaction vessel.
The process affords microscopic
The microscopic scale () is the scale of objects and events smaller than those that can easily be seen by the naked eye, requiring a lens or microscope to see them clearly. In physics, the microscopic scale is sometimes regarded as the scale betwe ...
particles of colloidal silica
Colloidal silicas are suspensions of fine amorphous, nonporous, and typically spherical silica particles in a liquid phase. It may be produced by Stöber process from Tetraethyl orthosilicate (TEOS).
Properties
Usually they are suspended in an ...
with diameters ranging from 50 to 2000 nm; particle size
Particle size is a notion introduced for comparing dimensions of solid particles ('' flecks''), liquid particles ('' droplets''), or gaseous particles ('' bubbles''). The notion of particle size applies to particles in colloids, in ecology, in ...
s are fairly uniform with the distribution Distribution may refer to:
Mathematics
*Distribution (mathematics), generalized functions used to formulate solutions of partial differential equations
*Probability distribution, the probability of a particular value or value range of a varia ...
determined by the choice of conditions such as reactant concentrations, catalysts, and temperature. Larger particles are formed when the concentrations of water and ammonia are raised, but with a consequent broadening of the particle-size distribution. The initial concentration of TEOS is inversely proportional to the size of the resulting particles; thus, higher concentrations on average lead to smaller particles due to the greater number of nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
sites, but with a greater spread of sizes. Particles with irregular shapes can result when the initial precursor concentration is too high. The process is temperature-dependent, with cooling (and hence slower reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
s) leading to a monotonic increase in average particle size, but control distribution cannot be maintained at overly low temperatures.
Two-step process
In 1999 Cédric Boissière and his team developed a two-step process whereby the hydrolysis at low pH (1 – 4) is completed before the condensation reaction is initiated by the addition ofsodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes ...
(NaF). The two-step procedure includes the addition of a nonionic surfactant template to ultimately produce mesoporous silica
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (50 ...
particles. The main advantage of sequencing the hydrolysis and condensation reactions is the ability to ensure complete homogeneity
Homogeneity and heterogeneity are concepts relating to the Uniformity (chemistry), uniformity of a Chemical substance, substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, ...
of the surfactant and the precursor TEOS mixture. Consequently, the diameter and shape of the product particles as well as the pore size are determined solely by the reaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a ...
and the quantity of sodium fluoride introduced; higher relative fluoride levels produces a greater number of nucleation sites and hence smaller particles. Decoupling the hydrolysis and condensation process affords a level of product control that is substantially superior to that afforded by the one-step Stöber process, with particle size controlled nearly completely by the sodium fluoride-to-TEOS ratio.
The two-step Stöber process begins with a mixture of TEOS, water, alcohol, and a nonionic surfactant, to which hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
is added to produce a microemulsion. This solution is allowed to stand until hydrolysis is complete, much like in the one-step Stöber process but with the hydrochloric acid replacing the ammonia as catalyst. Sodium fluoride is added to the resulting homogeneous solution, initiating the condensation reaction by acting as nucleation seed. The silica particles are collected by filtration and calcined to remove the nonionic surfactant template by combustion, resulting in the mesoporous silica product.
The selection of conditions for the process allows for control of pore sizes, particle diameter, and their distributions, as in the case of the one-step approach. Porosity in the modified process is controllable through the introduction of a swelling agent, the choice of temperature, and the quantity of sodium fluoride catalyst added. A swelling agent (such as mesitylene) causes increases in volume and hence in pore size, often by solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
absorption, but is limited by the solubility of the agent in the system. Pore size varies directly with temperature, bound by the lower out of the surfactant cloud point and the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of water. Sodium fluoride concentration produces direct but non-linear changes in porosity, with the effect decreasing as the added fluoride concentration tends to an upper limit.
Kinetics
The LaMer model for the kinetics of the formation of hydrosols is widely applicable for production of monodisperse systems, and it was originally hypothesized that the Stöber process followed thismonomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
addition model. This model includes a rapid burst of nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
forming all of the particle growth sites, then proceeds with hydrolysis as the rate-limiting step for condensation of triethylsilanol monomers to the nucleation sites. The production of monodisperse particle sizes is attributed to monomer addition happening at a slower rate on larger particles as a consequence of diffusion-limited mass transfer of TEOS. However, experimental evidence demonstrates that the concentration of hydrolyzed TEOS stays above that required for nucleation until late into the reaction, and the introduction of seeded growth nuclei does not match the kinetics of a monomer addition process. Consequently, the LaMer model has been rejected in favour of a kinetic model based around growth via particle aggregation
Particle agglomeration refers to the formation of assemblages in a suspension (chemistry), suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid ...
.
Under an aggregation-based model, nucleation sites are continually being generated and absorbed where the merging leads to particle growth. The generation of the nucleation sites and the interaction energy between merging particles dictates the overall kinetics of the reaction. The generation of the nucleation sites follows the equation below:
:
where ''J'' is the nucleation rate, ''k''1 and ''k''2 are rate constants based on the concentrations of H2O and NH3 and ''gs'' is the normalization factor based on the amount of silica precursor. Adjusting the concentration ratios of these compounds directly influences the rate at which nucleation sites are produced.
Merging of nucleation sites between particles is influenced by their interaction energies. The total interaction energy is dependent on three forces: electrostatic
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), mean ...
repulsion of like charges, vanderWaals attraction between particles, and the effects of solvation
Solvations describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, includi ...
. These interaction energies (equations below) describe the particle aggregation process and demonstrate why the Stöber process produces particles that are uniform in size.
:
The van der Waals attraction forces are governed by the following equation:
: