Staurosporine 2 on:
[Wikipedia]
[Google]
[Amazon]
Staurosporine (antibiotic AM-2282 or STS) is a
 This is followed by a
This is followed by a
natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
originally isolated in 1977 from the bacterium '' Streptomyces staurosporeus''.
It was the first of over 50 alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s that were discovered to share this type of bis-indole chemical structure. The chemical structure of staurosporine was elucidated by X-ray crystalography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract in specific directions. By measuring the angles and ...
in 1994.
Staurosporine was discovered to have biological activities ranging from anti-fungal to anti-hypertensive.
The interest in these activities resulted in a large investigative effort in chemistry and biology and the discovery of the potential for anti-cancer treatment.
Biological activities
The main biological activity of staurosporine is theinhibition
Inhibitor or inhibition may refer to:
Biology
* Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity
* Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotransm ...
of protein kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them ( phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a f ...
s through the prevention of ATP binding to the kinase. This is achieved through the stronger affinity of staurosporine to the ATP-binding site on the kinase. Staurosporine is a prototypical ATP-competitive kinase inhibitor in that it binds to many kinases with high affinity, though with little selectivity. Structural analysis of kinase pockets demonstrated that main chain atoms which are conserved in their relative positions to staurosporine contributes to staurosporine promiscuity. This lack of specificity has precluded its clinical use, but has made it a valuable research tool. In research, staurosporine is used to induce apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
. The mechanism of how it mediates this is not well understood. It has been found that one way in which staurosporine induces apoptosis is by activating caspase-3
Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the ''CASP3'' gene. ''CASP3'' orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also ...
. At lower concentration, depending on the cell type, staurosporine induces specific cell cycle effects arresting cells either in G1 or in G2 phase of the cell cycle.
Chemistry family
Staurosporine is anindolocarbazole
Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer as well as antimicrobial drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolat ...
. It belongs to the most frequently isolated group of indolocarbazoles: Indolo(2,3-a)carbazoles. Of these, Staurosporine falls within the most common subgroup, called Indolo(2,3-a)pyrrole(3,4-c)carbazoles. These fall into two classes - halogenated (chlorinated) and non-halogenated. Halogenated indolo(2,3-a)pyrrole(3,4-c)carbazoles have a fully oxidized C-7 carbon with only one indole nitrogen containing a β-glycosidic bond, while non-halogenated indolo(2,3-a)pyrrole(3,4-c)carbazoles have both indole nitrogens glycosylated, and a fully reduced C-7 carbon. Staurosporine is in the non-halogenated class.
Staurosporine is the precursor of the novel protein kinase inhibitor
A protein kinase inhibitor (PKI) is a type of enzyme inhibitor that blocks the action of one or more protein kinases. Protein kinases are enzymes that phosphorylate (add a phosphate, or PO4, group) to a protein and can modulate its function.
The ...
midostaurin
Midostaurin, sold under the brand name Rydapt by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocyt ...
(PKC412). Besides midostaurin, staurosporine is also used as a starting material in the commercial synthesis of K252c
K, or k, is the eleventh letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''kay'' (pronounced ), plural ''kays''.
The letter ...
(also called staurosporine aglycone). In the natural biosynthetic pathway, K252c is a precursor of staurosporine.
Biosynthesis
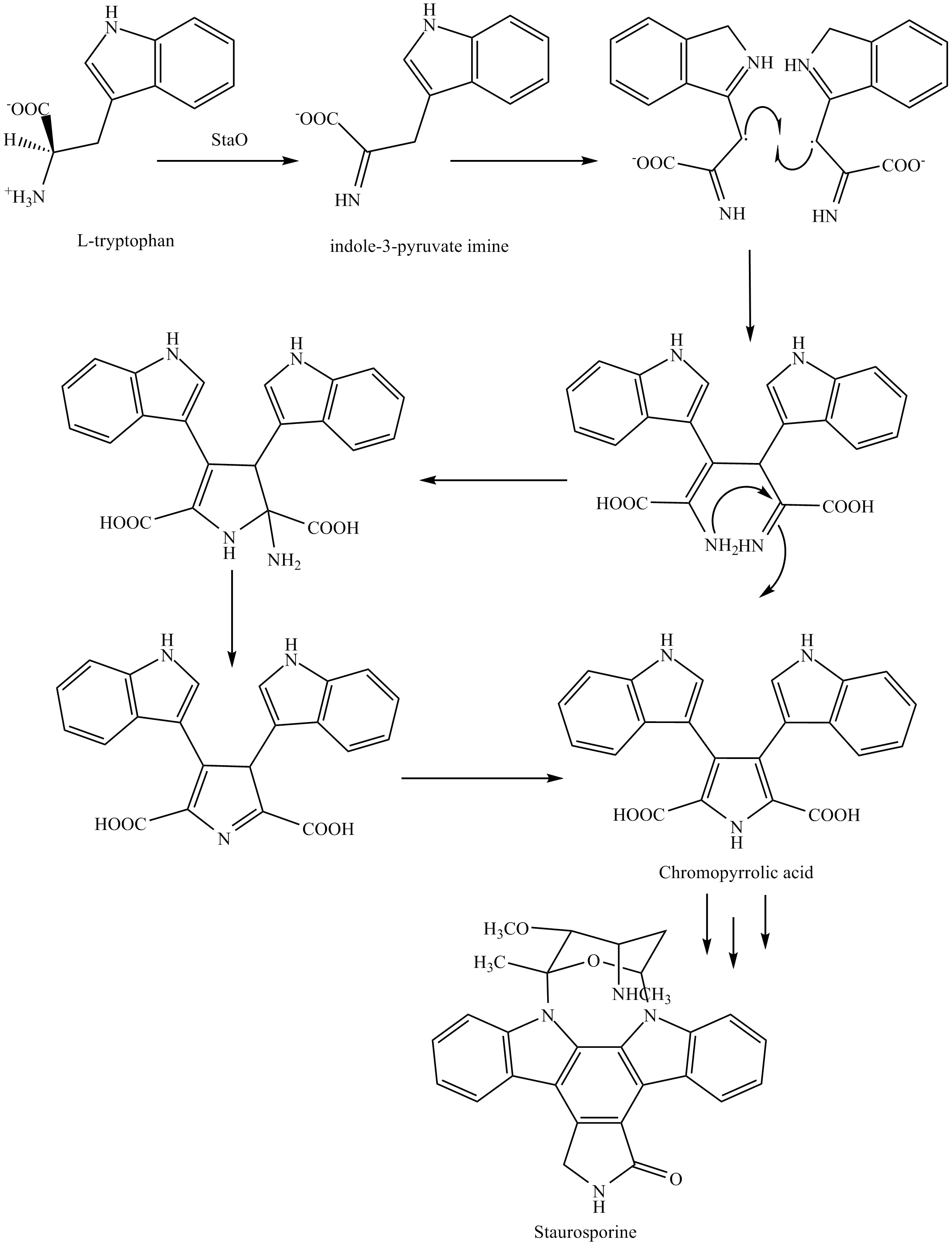
The biosynthesis of staurosporine starts with the amino acid L-tryptophan in itszwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups.
:
(1,2- dipolar compounds, such as ylides, are sometimes excluded from ...
ic form. Tryptophan is converted to an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
by enzyme StaO which is an L-amino acid oxidase (that may be FAD dependent). The imine is acted upon by StaD to form an uncharacterized intermediate proposed to be the dimerization product between 2 imine molecules. Chromopyrrolic acid is the molecule formed from this intermediate after the loss of VioE (used in the biosynthesis of violacein
Violacein is a naturally-occurring bis-indole pigment with antibiotic (anti-bacterial, anti-viral, anti-fungal and anti-tumor) properties. Violacein is produced by several species of bacteria, including ''Chromobacterium violaceum'', and gives th ...
– a natural product formed from a branch point in this pathway that also diverges to form rebeccamycin
Rebeccamycin (NSC 655649) is a weak topoisomerase I inhibitor isolated from ''Nocardia'' bacteria. It is structurally similar to staurosporine, but does not show any inhibitory activity against protein kinases. It shows significant antitumor prope ...
. An aryl aryl coupling thought to be catalyzed by a cytochrome P450 enzyme to form an aromatic ring system occurs.
 This is followed by a
This is followed by a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
between the indole nitrogens resulting in cyclization and then decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
assisted by StaC exclusively forming staurosporine aglycone or K252c. Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
is transformed to NTP-L-ristoamine by StaA/B/E/J/I/K which is then added on to the staurosporine aglycone at 1 indole N by StaG. The StaN enzyme reorients the sugar by attaching it to the 2nd indole nitrogen into an unfavored conformation to form intermediated O-demethyl-N-demethyl-staurosporine. Lastly, O-methylation of the 4'amine by StaMA and N-methylation of the 3'-hydroxy by StaMB leads to the formation of staurosporine.
Research in preclinical use
When encapsulated inliposome
A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug deliver ...
nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
, staurosporine is shown to suppress tumors ''in vivo'' in a mouse model without the toxic side effects which have prohibited its use as an anti-cancer drug with high apoptotic activity. Researchers in UC San Diego Moores Cancer Center
The Rebecca and John Moores Cancer Center is the region's only NCI-designated Cancer Center, part of UC San Diego Health and affiliated with the University of California, San Diego. It is supported, in part, by the National Cancer Institute.
His ...
develop a platform technology of high drug-loading efficiency by manipulating the pH environment of the cells. When injected into the mouse glioblastoma
Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nons ...
model, staurosporine is found to accumulate primarily in the tumor via fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
confirmation, and the mice did not suffer weight loss compared to the control mice administered with the free compound, an indicator of reduced toxicity.
List of compounds closely related to Staurosporine
*K252a
K252a is an alkaloid isolated from ''Nocardiopsis'' bacteria. This staurosporine analog is a highly potent cell permeable enzyme inhibitor, inhibitor of CaM kinase and phosphorylase kinase (IC50 = 1.8 and 1.7 nanomole, nmol/liter, L, respectively ...
* Stauprimide
Stauprimide is a semi-synthetic analog of the staurosporine family of indolocarbazoles. Stauprimide was first published in 1994 as part of an extensive structure-activity investigation to improve the selective inhibition of protein kinase C as a p ...
* Midostaurin
Midostaurin, sold under the brand name Rydapt by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocyt ...
References
{{reflist Bacterial alkaloids Antibiotics Gamma-lactams Protein kinase inhibitors Indolocarbazoles