Skeletal model on:
[Wikipedia]
[Google]
[Amazon]
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an
 It does not matter which end of the chain one starts numbering from, as long as consistency is maintained when drawing diagrams. The condensed formula or the IUPAC name will confirm the orientation. Some molecules will become familiar regardless of the orientation.
It does not matter which end of the chain one starts numbering from, as long as consistency is maintained when drawing diagrams. The condensed formula or the IUPAC name will confirm the orientation. Some molecules will become familiar regardless of the orientation.


Image:Stereochemistry-example-3D-balls.png,
(''R'')-2-chloro-2-fluoropentane
Image:(R)-2-Chloro-2-fluoropentane.svg,
The relevant chemical bonds can be depicted in several ways:
* Solid lines represent bonds in the plane of the paper or screen.
* Solid wedges represent bonds that point out of the plane of the paper or screen, towards the observer.
* Hashed wedges or dashed lines (thick or thin) represent bonds that point into the plane of the paper or screen, away from the observer.
* Wavy lines represent either unknown stereochemistry or a mixture of the two possible stereoisomers at that point.
*An obsolescent depiction of hydrogen stereochemistry that used to be common in 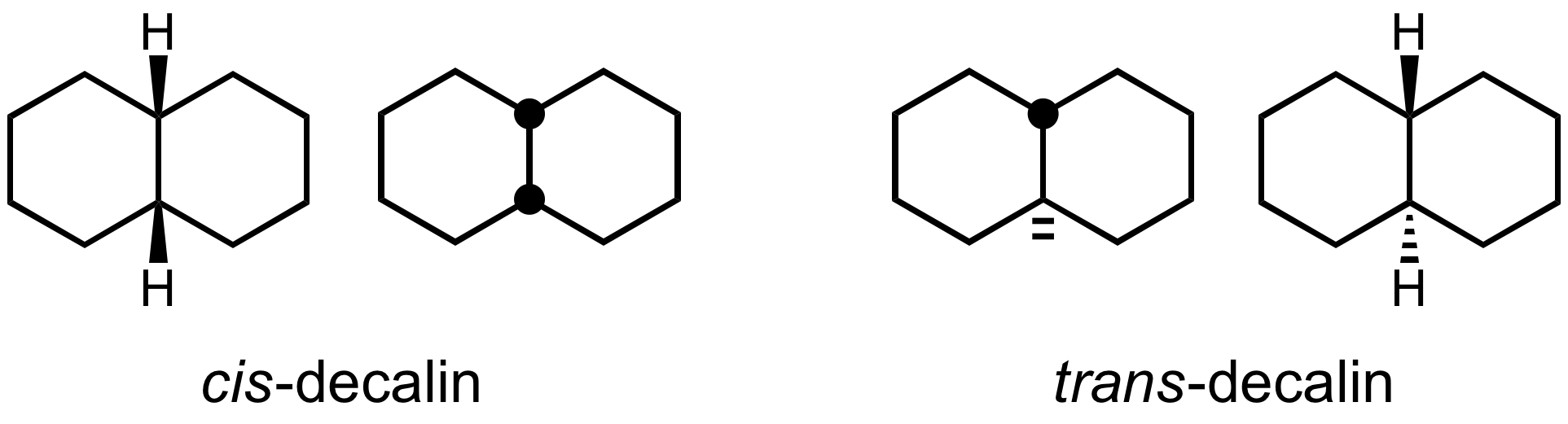 An early use of this notation can be traced back to
An early use of this notation can be traced back to 

Drawing organic molecules
from ''chemguide.co.uk'' {{Visualization Organic chemistry Chemical formulas Chemical structures
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
is a type of minimalist structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is al ...
representing a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
's atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
, bonds and some details of its geometry
Geometry (; ) is a branch of mathematics concerned with properties of space such as the distance, shape, size, and relative position of figures. Geometry is, along with arithmetic, one of the oldest branches of mathematics. A mathematician w ...
. The lines in a skeletal formula represent bonds between carbon atoms, unless labelled with another element. Labels are optional for carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms, and the hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atoms attached to them.
An early form of this representation was first developed by organic chemist August Kekulé
Friedrich August Kekulé, later Friedrich August Kekule von Stradonitz ( , ; 7 September 1829 – 13 July 1896), was a German organic chemist. From the 1850s until his death, Kekulé was one of the most prominent chemists in Europe, especially ...
, while the modern form is closely related to and influenced by the Lewis structure
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the chemical bond, bonding between atoms of a molecule, as well as the lone pairs of elec ...
of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulas have become ubiquitous in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, partly because they are relatively quick and simple to draw, and also because the curved arrow notation used for discussions of reaction mechanisms and electron delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
can be readily superimposed.
Several other ways of depicting chemical structures are also commonly used in organic chemistry (though less frequently than skeletal formulae). For example, conformational structures look similar to skeletal formulae and are used to depict the approximate positions of atoms in 3D space, as a perspective drawing. Other types of representation, such as Newman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
, Haworth projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. A Haworth projection approximates the shapes of the actual mole ...
or Fischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates a ...
, also look somewhat similar to skeletal formulae. However, there are slight differences in the conventions used, and the reader needs to be aware of them in order to understand the structural details encoded in the depiction. While skeletal and conformational structures are also used in organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
and inorganic chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subj ...
, the conventions employed also differ somewhat.
The skeleton
Terminology
The skeletal structure of an organic compound is the series of atoms bonded together that form the essential structure of the compound. The skeleton can consist of chains, branches and/or rings of bonded atoms. Skeletal atoms other than carbon or hydrogen are calledheteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
s.
The skeleton has hydrogen and/or various substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s bonded to its atoms. Hydrogen is the most common non-carbon atom that is bonded to carbon and, for simplicity, is not explicitly drawn. In addition, carbon atoms are not generally labelled as such directly (i.e. with "C"), whereas heteroatoms are always explicitly noted as such ("N" for nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, "O" for oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, etc.)
Heteroatoms and other groups of atoms that give rise to relatively high rates of chemical reactivity, or introduce specific and interesting characteristics in the spectra of compounds are called functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s, as they give the molecule a function. Heteroatoms and functional groups are collectively called "substituents", as they are considered to be a substitute for the hydrogen atom that would be present in the parent hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
of the organic compound.
Basic structure
As in Lewis structures, covalent bonds are indicated by line segments, with a doubled or tripled line segment indicatingdouble
Double, The Double or Dubble may refer to:
Mathematics and computing
* Multiplication by 2
* Double precision, a floating-point representation of numbers that is typically 64 bits in length
* A double number of the form x+yj, where j^2=+1
* A ...
or triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
ing, respectively. Likewise, skeletal formulae indicate formal charges
In chemistry, a formal charge (F.C. or ), in the covalent view of Chemical bond, chemical bonding, is the hypothetical electric charge, charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally bet ...
associated with each atom, with lone pairs usually being optional . In fact, skeletal formulae can be thought of as abbreviated Lewis structures that observe the following simplifications:
*Carbon atoms are represented by the vertices (intersections or termini) of line segments. For clarity, methyl groups are often explicitly written out as Me or CH3, while (hetero)cumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumu ...
carbons are frequently represented by a heavy center dot.
* Hydrogen atoms attached to carbon are implied. An unlabeled vertex is understood to represent a carbon attached to the number of hydrogens required to satisfy the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
, while a vertex labeled with a formal charge and/or nonbonding electron(s) is understood to have the number of hydrogen atoms required to give the carbon atom these indicated properties. Optionally, acetylenic and formyl hydrogens can be shown explicitly for the sake of clarity.
* Hydrogen atoms attached to a heteroatom are shown explicitly. The heteroatom and hydrogen atoms attached thereto are usually shown as a single group (e.g., OH, NH2) without explicitly showing the hydrogen–heteroatom bond. Heteroatoms with simple alkyl or aryl substituents, like methoxy (OMe) or dimethylamino (NMe2), are sometimes shown in the same way, by analogy.
* Lone pairs on carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
carbons must be indicated explicitly while lone pairs in other cases are optional and are shown only for emphasis. In contrast, formal charges and unpaired electrons on main-group elements are always explicitly shown.
In the standard depiction of a molecule, the canonical form
In mathematics and computer science, a canonical, normal, or standard form of a mathematical object is a standard way of presenting that object as a mathematical expression. Often, it is one which provides the simplest representation of an obje ...
(resonance structure) with the greatest contribution is drawn. However, the skeletal formula is understood to represent the "real molecule" that is, the weighted average of all contributing canonical forms. Thus, in cases where two or more canonical forms contribute with equal weight (e.g., in benzene, or a carboxylate anion) and one of the canonical forms is selected arbitrarily, the skeletal formula is understood to depict the true structure, containing equivalent bonds of fractional order, even though the delocalized bonds are depicted as nonequivalent single and double bonds.
Contemporary graphical conventions
Since skeletal structures were introduced in the latter half of the 19th century, their appearance has undergone considerable evolution. The graphical conventions in use today date to the 1980s. Thanks to the adoption of the ChemDraw software package as a ''de facto'' industry standard (byAmerican Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all ...
, Royal Society of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the ...
, and Gesellschaft Deutscher Chemiker
The German Chemical Society () is a learned society and professional association founded in 1949 to represent the interests of German chemists in local, national and international contexts. GDCh "brings together people working in chemistry and th ...
publications, for instance), these conventions have been nearly universal in the chemical literature since the late 1990s. A few minor conventional variations, especially with respect to the use of stereobonds, continue to exist as a result of differing US, UK and European practice, or as a matter of personal preference. As another minor variation between authors, formal charges can be shown with the plus or minus sign in a circle (⊕, ⊖) or without the circle. The set of conventions that are followed by most authors is given below, along with illustrative examples.
Implicit carbon and hydrogen atoms
For example, the skeletal formula ofhexane
Hexane () or ''n''-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
Hexane is a colorless liquid, odorless when pure, and with a boiling point of approximately . It is widely used as ...
(top) is shown below. The carbon atom labeled C1 appears to have only one bond, so there must also be three hydrogens bonded to it, in order to make its total number of bonds four. The carbon atom labelled C3 has two bonds to other carbons and is therefore bonded to two hydrogen atoms as well. A Lewis structure
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the chemical bond, bonding between atoms of a molecule, as well as the lone pairs of elec ...
(middle) and ball-and-stick model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the Molecular geometry, three-dimensional position of the atoms and the chemical bond, bonds between them. The atoms are typically represente ...
(bottom) of the actual molecular structure of hexane, as determined by X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, are shown for comparison.
 It does not matter which end of the chain one starts numbering from, as long as consistency is maintained when drawing diagrams. The condensed formula or the IUPAC name will confirm the orientation. Some molecules will become familiar regardless of the orientation.
It does not matter which end of the chain one starts numbering from, as long as consistency is maintained when drawing diagrams. The condensed formula or the IUPAC name will confirm the orientation. Some molecules will become familiar regardless of the orientation.
Explicit heteroatoms and hydrogen atoms
All atoms that are not carbon or hydrogen are signified by theirchemical symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist ...
, for instance Cl for chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, O for oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, Na for sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, and so forth. In the context of organic chemistry, these atoms are commonly known as heteroatoms (the prefix
A prefix is an affix which is placed before the stem of a word. Particularly in the study of languages, a prefix is also called a preformative, because it alters the form of the word to which it is affixed.
Prefixes, like other affixes, can b ...
''hetero-'' comes from Greek ''ἕτερος'' héteros, meaning "other").
Any hydrogen atoms bonded to heteroatoms ''are'' drawn explicitly. In ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, C2H5OH, for instance, the hydrogen atom bonded to oxygen is denoted by the symbol H, whereas the hydrogen atoms which are bonded to carbon atoms are not shown directly.
Lines representing heteroatom-hydrogen bonds are usually omitted for clarity and compactness, so a functional group like the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group is most often written −OH instead of −O−H. These bonds are sometimes drawn out in full in order to accentuate their presence when they participate in reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
s.
Shown below for comparison are a skeletal formula (top), its Lewis structure
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the chemical bond, bonding between atoms of a molecule, as well as the lone pairs of elec ...
(middle) and its ball-and-stick model (bottom) of the actual 3D structure of the ethanol molecule in the gas phase, as determined by microwave spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.
History
The ammonia molecule NH3 is shaped like a pyramid 0.38 Å in height, with an equilatera ...
.

Pseudoelement symbols
There are also symbols that appear to be chemical element symbols, but represent certain very common substituents or indicate an unspecified member of a group of elements. These are called pseudoelement symbols or organic elements and are treated like univalent "elements" in skeletal formulae. A list of common pseudoelement symbols:General symbols
*X for any (pseudo
Pseudo- (from , ) is a prefix used in a number of languages, often to mark something as a fake or insincere version.
In English, the prefix is used on both nouns and adjectives. It can be considered a privative prefix specifically denoting '' ...
)halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
atom (in the related MLXZ notation, X represents a one-electron donor ligand)
*L ''or'' L''n'' for a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
or ligands (in the related MLXZ notation, L represents a two-electron donor ligand)
*M ''or'' Met for any metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
atom ( is used to indicate a ligated metal, ML''n'', when the identities of the ligands are unknown or irrelevant)
*E ''or'' El for any electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
(in some contexts, E is also used to indicate any p-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-bl ...
element)
*Nu for any nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
*Z for conjugating electron-withdrawing groups (in the related MLXZ notation, Z represents a zero-electron donor ligand; ''in unrelated usage, Z is also an abbreviation for the carboxybenzyl group''.)
*D for deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
(2H)
*T for tritium
Tritium () or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with a half-life of ~12.33 years. The tritium nucleus (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the ...
(3H)
Alkyl groups
*R for anyalkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
group or even any organyl
In organic and organometallic chemistry, an organyl group (commonly denoted by the letter " R") is an organic substituent with one (sometimes more) free valence electron(s) at a carbon atom.. The term is often used in chemical patent literatur ...
group (Alk can be used to unambiguously indicate an alkyl group)
*Me for the methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated a ...
*Et for the ethyl group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied ...
*Pr, ''n''-Pr, ''or'' ''n''Pr for the (''normal'') propyl group (''Pr is also the symbol for the element praseodymium
Praseodymium is a chemical element; it has symbol Pr and atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic ...
. However, since the propyl group is monovalent, while praseodymium is nearly always trivalent, ambiguity rarely, if ever, arises in practice.'')
*''i''-Pr ''or'' ''i''Pr for the isopropyl
In organic chemistry, a propyl group is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent ...
group
*All for the allyl group (uncommon)
*Bu, ''n''-Bu ''or'' ''n''Bu for the (''normal'') butyl group
*''i''-Bu ''or'' ''i''Bu (''i'' often italicized) for the isobutyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
group
*''s''-Bu ''or'' ''s''Bu for the ''secondary'' butyl group
*''t''-Bu ''or'' ''t''Bu for the ''tertiary'' butyl group
*Pn for the pentyl group (''or'' Am for the synonymous amyl
Amyl may refer to:
* Amylum or starch, a carbohydrate
** Amylopectin, a polymer of glucose found in plants; one of two components of starch
** Amylose, a helical polymer made of α-D-glucose units; one of two components of starch
* Pentyl, a fiv ...
group, ''although Am is also the symbol for americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
.'')
*Np ''or'' Neo for the neopentyl group (''Warning: Organometallic chemists often use Np for the related neophyl group, PhMe2C–. Np is also the symbol for the element neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar Syste ...
.'')
*Cy ''or'' Chx for the cyclohexyl group
*Ad for the 1-adamantyl
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the mo ...
group
*Tr ''or'' Trt for the trityl
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the chemical formula, formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic sk ...
group
Aromatic and unsaturated substituents
*Ar for anyaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
substituent ''(Ar is also the symbol for the element argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
. However, argon is inert under all usual conditions encountered in organic chemistry, so the use of Ar to represent an aryl substituent never causes confusion.)''
*Het for any heteroaromatic substituent
*Bn ''or'' Bzl for the benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
group (''not to be confused with Bz for benzoyl group; However, old literature may use Bz for benzyl group.'')
*Dipp for the 2,6-diisopropylphenyl group
*Mes for the mesityl group
*Ph, Φ, ''or'' φ for the phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
(''the use of phi
Phi ( ; uppercase Φ, lowercase φ or ϕ; ''pheî'' ; Modern Greek: ''fi'' ) is the twenty-first letter of the Greek alphabet.
In Archaic and Classical Greek (c. 9th to 4th century BC), it represented an aspirated voiceless bilabial plos ...
for phenyl has been in decline'')
*Tol for the tolyl group, usually the ''para'' isomer
*Is ''or'' Tipp for the 2,4,6-triisopropylphenyl group (''the former symbol is derived from the synonym'' ''isityl'')
*An for the anisyl group, usually the ''para'' isomer (''An is also the symbol for a generic actinoid element. However, since the anisyl group is monovalent, while the actinides are usually divalent, trivalent, or even higher valency, ambiguity rarely, if ever, arises in practice.'')
*Cp for the cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
group (''Cp was the symbol for cassiopeium, a former name for lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
'')
*Cp* for the pentamethylcyclopentadienyl group
*Vi for the vinyl group (uncommon)
Functional groups
*Ac for theacetyl
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
group ''(Ac is also the symbol for the element actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
. However, actinium is almost never encountered in organic chemistry, so the use of Ac to represent the acetyl group never causes confusion)'';
*Bz for the benzoyl group; OBz is the benzoate
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
group
*Piv for the pivalyl (''t''-butylcarbonyl) group; OPiv is the pivalate group
*Bt for the 1-benzotriazolyl group
*Im for the 1-imidazolyl group
*NPhth for the phthalimide-1-yl group
Sulfonyl/sulfonate groups
Sulfonate esters are oftenleaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
s in nucleophilic substitution reactions. ''See the articles on sulfonyl
In organosulfur chemistry, a sulfonyl group is either a functional group found primarily in sulfones, or a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups.
Group
Sulfonyl groups can be w ...
and sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
groups for further information.''
*Bs for the brosyl (''p''-bromobenzenesulfonyl) group; OBs is the brosylate group
*Ms for the mesyl (methanesulfonyl) group; OMs is the mesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the gr ...
group
*Ns for the nosyl (''p''-nitrobenzenesulfonyl) group ''(Ns was the chemical symbol for nielsbohrium, but that was renamed bohrium
Bohrium is a synthetic chemical element; it has symbol Bh and atomic number 107. It is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in particle accelerators but is not found in nature. All known isotopes of ...
, Bh)''; ONs is the nosylate group
*Tf for the triflyl
In organic chemistry, the triflyl group (Preferred IUPAC name, systematic name: trifluoromethanesulfonyl group) is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflyl group is often represented b ...
(trifluoromethanesulfonyl) group; OTf is the triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
group
*Nf for the nonaflyl (nonafluorobutanesulfonyl) group, ; ONf is the nonaflate group
*Ts for tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
(''p-''toluenesulfonyl) group ''(Ts is also the symbol for the element tennessine
Tennessine is a synthetic element; it has Chemical symbol, symbol Ts and atomic number 117. It has the second-highest atomic number and joint-highest atomic mass of all known elements and is the penultimate element of the Period 7 element, 7th ...
. However, tennessine is too unstable to ever be encountered in organic chemistry, so the use of Ts to represent tosyl never causes confusion)''; OTs is the tosylate group
Protecting groups
Aprotecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction, facilitating multistep organic synthesis.
*Boc for the ''t-''butoxycarbonyl group
*Cbz ''or'' Z for the carboxybenzyl
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily c ...
group
*Fmoc for the fluorenylmethoxycarbonyl group
*Alloc for the allyloxycarbonyl group
*Troc for the trichloroethoxycarbonyl group
*TMS, TBDMS, TES, TBDPS, TIPS, ... for various silyl ether groups
*PMB for the 4-methoxybenzyl group
*MOM for the methoxymethyl group
*THP for the 2-tetrahydropyranyl group
Multiple bonds
Two atoms can be bonded by sharing more than one pair of electrons. The common bonds to carbon are single, double and triple bonds. Single bonds are most common and are represented by a single, solid line between two atoms in a skeletal formula. Double bonds are denoted by two parallel lines, and triple bonds are shown by three parallel lines. In more advanced theories of bonding, non-integer
An integer is the number zero (0), a positive natural number (1, 2, 3, ...), or the negation of a positive natural number (−1, −2, −3, ...). The negations or additive inverses of the positive natural numbers are referred to as negative in ...
values of bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
exist. In these cases, a combination of solid and dashed lines indicate the integer and non-integer parts of the bond order, respectively.
Benzene rings
In recent years,benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
is generally depicted as a hexagon with alternating single and double bonds, much like the structure Kekulé originally proposed in 1872. As mentioned above, the alternating single and double bonds of "1,3,5-cyclohexatriene" are understood to be a drawing of one of the two equivalent canonical forms of benzene (the one explicitly shown and the one with the opposite pattern of formal single and double bonds), in which all carbon–carbon bonds are of equivalent length and have a bond order of exactly 1.5. For aryl rings in general, the two analogous canonical forms are almost always the primary contributors to the structure, but they are nonequivalent, so one structure may make a slightly greater contribution than the other, and bond orders may differ somewhat from 1.5.
An alternate representation that emphasizes this delocalization uses a circle, drawn inside the hexagon of single bonds, to represent the delocalized pi orbital
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occ ...
. This style, based on one proposed by Johannes Thiele Johannes Thiele may refer to:
*Johannes Thiele (zoologist)
*Johannes Thiele (chemist)
{{hndis, Thiele, Johannes ...
, used to be very common in introductory organic chemistry textbooks and is still frequently used in informal settings. However, because this depiction does not keep track of electron pairs and is unable to show the precise movement of electrons, it has largely been superseded by the Kekuléan depiction in pedagogical and formal academic contexts.
Stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
is conveniently denoted in skeletal formulae:
Ball-and-stick model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the Molecular geometry, three-dimensional position of the atoms and the chemical bond, bonds between them. The atoms are typically represente ...
of (''R'')-2-chloro-2-fluoropentane
Skeletal formula of
(''R'')-2-chloro-2-fluoropentane
Image:(S)-2-Chloro-2-fluoropentane.svg, (''R'')-2-chloro-2-fluoropentane
Skeletal formula of
(''S'')-2-chloro-2-fluoropentane
Image:Amphetamine-2D-skeletal.svg, (''S'')-2-chloro-2-fluoropentane
Skeletal formula of
amphetamine
Amphetamine (contracted from Alpha and beta carbon, alpha-methylphenethylamine, methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, an ...
, indicating a mixture of two stereoisomers: (''R'')- and (''S'')-steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes t ...
chemistry is the use of a filled circle centered on a vertex (sometimes called H-dot/H-dash/H-circle, respectively) for an upward pointing hydrogen atom and two hash marks next to vertex or a hollow circle for a downward pointing hydrogen atom.  An early use of this notation can be traced back to
An early use of this notation can be traced back to Richard Kuhn
Richard Johann Kuhn (; 3 December 1900 – 31 July 1967) was an Austrian-German biochemist who was awarded the Nobel Prize in Chemistry in 1938 "for his work on carotenoids and vitamins".
Biography
Early life
Kuhn was born in Vienna, Austria ...
who in 1932 used solid thick lines and dotted lines in a publication. The modern solid and hashed wedges were introduced in the 1940s by Giulio Natta
Giulio Natta (; 26 February 1903 – 2 May 1979) was an Italian chemical engineer and Nobel laureate. He won a Nobel Prize in Chemistry in 1963 with Karl Ziegler for work on high density polymers. He also received a Lomonosov Gold Medal in 19 ...
to represent the structure of high polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s, and extensively popularised in the 1959 textbook ''Organic Chemistry'' by Donald J. Cram and George S. Hammond.
Skeletal formulae can depict ''cis'' and ''trans'' isomers of alkenes. Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedral stereocenters). A crossed double-bond has been used sometimes; it is no longer considered an acceptable style for general use but may still be required by computer software.
Hydrogen bonds
Hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s are generally denoted by dotted or dashed lines. In other contexts, dashed lines may also represent partially formed or broken bonds in a transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
.
Notes
References
External links
Drawing organic molecules
from ''chemguide.co.uk'' {{Visualization Organic chemistry Chemical formulas Chemical structures