|
Sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non- oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: : An alternative is the condensation of a sulfonyl ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonate Anion Tet
In organosulfur chemistry, a sulfonate is a salt (chemistry), salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak base (chemistry), bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic organic reaction, preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: : An al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: : In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. In a related process, carboxyli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Triflate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula . It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence. Synthesis Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid. : Reactivity Hydrolysis Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol: : Methylation One ranking of methylating agents is . Methyl triflate will alkylate many functional groups which are very poor nucleophiles such as aldehydes, amides, and nitriles. It does not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
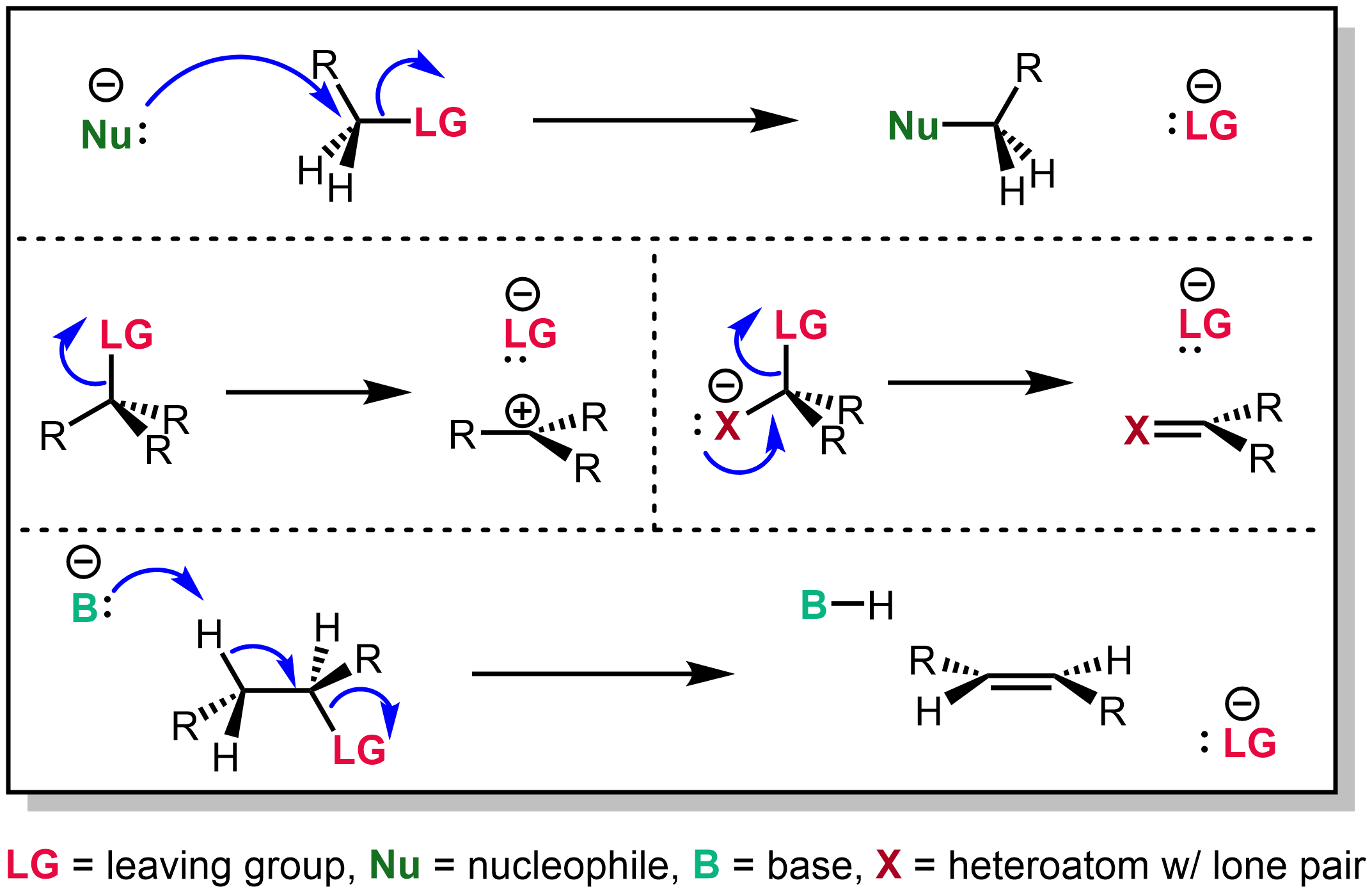
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium Triflate
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt (chemistry), salt consisting of scandium cations Sc3+ and triflate anions. Scandium triflate is used as a reagent in organic chemistry as a Lewis acid. Compared to other Lewis acids, this reagent is stable towards water and can often be used in organic reactions as a true catalyst rather than one used in stoichiometric amounts. The compound is prepared by reaction of scandium oxide with trifluoromethanesulfonic acid. An example of the scientific use of scandium triflate is the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% yield (chemistry), yield. : See also * Lanthanide trifluoromethanesulfonates References {{Scandium compounds Scandium compounds Triflates Acid catalysts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Chemistry
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane-1,3-sultone
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid. Synthesis It may be prepared by the acid catalyzed reaction of allyl alcohol and sodium bisulfite. Reactions 1,3-propane sultone is an activated ester and is susceptible to nucleophilic attack. It hydrolyzes to the 3-hydroxypropylsulfonic acid. : It has been used in the synthesis of specialist surfactants, such as CHAPS detergent. Safety Typical of activated esters, 1,3-propane sultone is an alkylating agent. 1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic. See also * 1,4-Butane sultone * Dimethyl sulfate * Vinylsulfonic acid * Isethionic acid * Sulfolane Sulfolane (also tetramethylene sulfone, IUPAC nomenclature, systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula . It is a colorless liquid commonly u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: \text + \text \; \ce \; \text + \text Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which said that any compound that can give a proton to another compound is an acid, and the compound that receives the proton is a base. A proton is a subatomic particle in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |