reaction center on:
[Wikipedia]
[Google]
[Amazon]
A photosynthetic reaction center is a complex of several
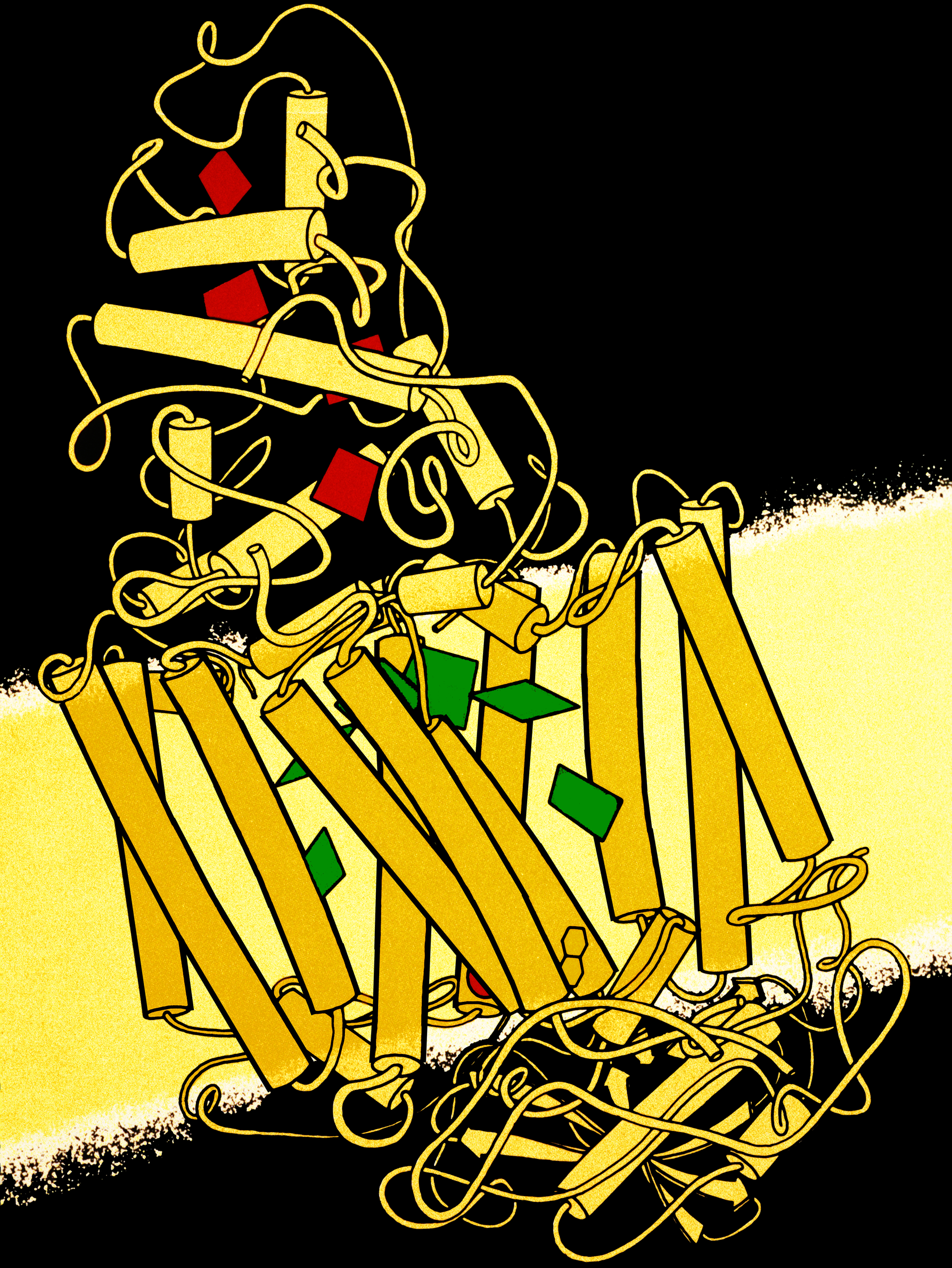
 The bacterial photosynthetic reaction center has been an important model to understand the structure and chemistry of the biological process of capturing light energy. In the 1960s, Roderick Clayton was the first to purify the reaction center complex from purple bacteria. However, the first crystal structure (upper image at right) was determined in 1984 by
The bacterial photosynthetic reaction center has been an important model to understand the structure and chemistry of the biological process of capturing light energy. In the 1960s, Roderick Clayton was the first to purify the reaction center complex from purple bacteria. However, the first crystal structure (upper image at right) was determined in 1984 by
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, biological pigment
A biological pigment, also known simply as a pigment or biochrome, is a substance produced by living organisms that have a color resulting from selective Absorption (electromagnetic radiation), color absorption. Biological pigments include plant ...
s, and other co-factors that together execute the primary energy conversion reactions of photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
s or pigments) such as chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
and pheophytin
Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of Photosystem II (PS II) in plants, and the type II photosynthetic reaction center (RC P870) found in ...
, as well as quinones. The energy of the photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
is used to excite an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
of a pigment. The free energy created is then used, via a chain of nearby electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents.
The electron accepting power of an electron acceptor is measured by its redox potential.
In the ...
s, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
towards carbon dioxide, eventually producing glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
. These electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
steps ultimately result in the conversion of the energy of photons to chemical energy.
Transforming light energy into charge separation
Reaction centers are present in all greenplant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s, algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
, and many bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
. A variety in light-harvesting complex
In biology, a light-harvesting complex or LHC is an aggregate consisting of proteins bound with chromophores (chlorophylls and carotenoids) that play a key role in photosynthesis. LHCs are arrayed around photosynthetic reaction centers in both pl ...
es exist across the photosynthetic species. Green plants and algae have two different types of reaction centers that are part of larger supercomplexes known as P700 in Photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the Light-dependent reactions, photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane ...
and P680 in Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem ...
. The structures of these supercomplexes are large, involving multiple light-harvesting complex
In biology, a light-harvesting complex or LHC is an aggregate consisting of proteins bound with chromophores (chlorophylls and carotenoids) that play a key role in photosynthesis. LHCs are arrayed around photosynthetic reaction centers in both pl ...
es. The reaction center found in '' Rhodopseudomonas'' bacteria is currently best understood, since it was the first reaction center of known structure and has fewer polypeptide chain
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty ami ...
s than the examples in green plants.
A reaction center is laid out in such a way that it captures the energy of a photon using pigment molecules and turns it into a usable form. Once the light energy has been absorbed directly by the pigment molecules, or passed to them by resonance transfer from a surrounding light-harvesting complex
In biology, a light-harvesting complex or LHC is an aggregate consisting of proteins bound with chromophores (chlorophylls and carotenoids) that play a key role in photosynthesis. LHCs are arrayed around photosynthetic reaction centers in both pl ...
, they release electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s into an electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
and pass energy to a hydrogen donor such as H2O to extract electrons and protons from it. In green plants, the electron transport chain has many electron acceptors including pheophytin
Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of Photosystem II (PS II) in plants, and the type II photosynthetic reaction center (RC P870) found in ...
, quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
, plastoquinone
Plastoquinone (PQ) is a terpenoid-quinone ( meroterpenoid) molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4- ...
, cytochrome bf, and ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
, which result finally in the reduced molecule NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require N ...
, while the energy used to split water results in the release of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. The passage of the electron through the electron transport chain also results in the pumping of protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' ( elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the pro ...
(hydrogen ions) from the chloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
's stroma and into the lumen, resulting in a proton gradient across the thylakoid membrane
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thyl ...
that can be used to synthesize ATP using the ATP synthase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed ...
molecule. Both the ATP and NADPH are used in the Calvin cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
to fix carbon dioxide into triose sugars.
Classification
Two classes of reaction centres are recognized. Type I, found in green-sulfur bacteria, Heliobacteria, and plant/cyanobacterial PS-I, use iron sulfur clusters as electron acceptors. Type II, found in chloroflexus, purple bacteria, and plant/cyanobacterial PS-II, use quinones. Not only do all members inside each class share common ancestry, but the two classes also, by means of common structure, appear related. Cyanobacteria, the precursor tochloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
s found in green plants, have both photosystems with both types of reaction centers. Combining the two systems allows for producing oxygen.
In purple bacteria (type II)
This section deals with the type II system found in purple bacteria.Structure

Hartmut Michel
Hartmut Michel (; born 18 July 1948) is a German biochemist, who received the 1988 Nobel Prize in Chemistry for determination of the first crystal structure of an integral membrane protein, a membrane-bound complex of proteins and co-factors that ...
, Johann Deisenhofer and Robert Huber for which they shared the Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
in 1988. This was also significant for being the first 3D crystal structure of any membrane protein complex.
Four different subunits were found to be important for the function of the photosynthetic reaction center. The L and M subunits, shown in blue and purple in the image of the structure, both span the lipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes form a continuous barrier around all cell (biology), cells. The cell membranes of almost all organisms and many viruses a ...
of the plasma membrane. They are structurally similar to one another, both having 5 transmembrane alpha helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
. Four bacteriochlorophyll b (BChl-b) molecules, two bacteriopheophytin b molecules (BPh) molecules, two quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
s (QA and QB), and a ferrous ion are associated with the L and M subunits. The H subunit, shown in gold, lies on the cytoplasmic side of the plasma membrane. A cytochrome subunit, not shown here, contains four c-type hemes and is located on the periplasmic surface (outer) of the membrane. The latter sub-unit is not a general structural motif in photosynthetic bacteria. The L and M subunits bind the functional and light-interacting cofactors, shown here in green.
Reaction centers from different bacterial species may contain slightly altered bacterio-chlorophyll and bacterio-pheophytin chromophores as functional co-factors. These alterations cause shifts in the colour of light that can be absorbed. The reaction center contains two pigments that serve to collect and transfer the energy from photon absorption: BChl and Bph. BChl roughly resembles the chlorophyll molecule found in green plants, but, due to minor structural differences, its peak absorption wavelength is shifted into the infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, with wavelengths as long as 1000 nm. Bph has the same structure as BChl, but the central magnesium ion is replaced by two protons. This alteration causes both an absorbance maximum shift and a lowered redox potential.
Mechanism
The process starts when light is absorbed by two BChl molecules that lie near theperiplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in Gram-negative (more accurately "diderm") bacteria. Using cryo-electron micros ...
ic side of the membrane. This pair of chlorophyll molecules, often called the "special pair", absorbs photons at 870 nm or 960 nm, depending on the species and, thus, is called P870 (for '' Rhodobacter sphaeroides'') or P960 (for '' Blastochloris viridis''), with ''P'' standing for "pigment"). Once P absorbs a photon, it ejects an electron, which is transferred through another molecule of Bchl to the BPh in the L subunit. This initial charge separation yields a positive charge on P and a negative charge on the BPh. This process takes place in 10 picoseconds (10−11 seconds).
The charges on the P+ and the BPh− could undergo charge recombination in this state, which would waste the energy and convert it into heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
. Several factors of the reaction center structure serve to prevent this. First, the transfer of an electron from BPh− to P960+ is relatively slow compared to two other redox reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
s in the reaction center. The faster reactions involve the transfer of an electron from BPh− (BPh− is oxidized to BPh) to the electron acceptor quinone (QA), and the transfer of an electron to P960+ (P960+ is reduced to P960) from a heme in the cytochrome subunit above the reaction center.
The high-energy electron that resides on the tightly bound quinone molecule QA is transferred to an exchangeable quinone molecule QB. This molecule is loosely associated with the protein and is fairly easy to detach. Two electrons are required to fully reduce QB to QH2, taking up two protons from the cytoplasm in the process. The reduced quinone QH2 diffuses through the membrane to another protein complex ( cytochrome bc1-complex) where it is oxidized. In the process the reducing power of the QH2 is used to pump protons across the membrane to the periplasmic space. The electrons from the cytochrome bc1-complex are then transferred through a soluble cytochrome c intermediate, called cytochrome c2, in the periplasm to the cytochrome subunit.
In Cyanobacteria and plants
Cyanobacteria, the precursor tochloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
s found in green plants, have both photosystems with both types of reaction centers. Combining the two systems allows for producing oxygen.
Oxygenic photosynthesis
In 1772, the chemistJoseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, Unitarian, Natural philosophy, natural philosopher, English Separatist, separatist theologian, Linguist, grammarian, multi-subject educator and Classical libera ...
carried out a series of experiments relating to the gases involved in respiration and combustion. In his first experiment, he lit a candle and placed it under an upturned jar. After a short period of time, the candle burned out. He carried out a similar experiment with a mouse
A mouse (: mice) is a small rodent. Characteristically, mice are known to have a pointed snout, small rounded ears, a body-length scaly tail, and a high breeding rate. The best known mouse species is the common house mouse (''Mus musculus'' ...
in the confined space of the burning candle. He found that the mouse died a short time after the candle had been extinguished. However, he could revivify the foul air by placing green plants in the area and exposing them to light. Priestley's observations were some of the first experiments that demonstrated the activity of a photosynthetic reaction center.
In 1779, Jan Ingenhousz
Jan Ingenhousz FRS (8 December 1730 – 7 September 1799) was a Dutch-British physiologist, biologist and chemist.
He is best known for discovering photosynthesis by showing that light is essential to the process by which green plants absorb ...
carried out more than 500 experiments spread out over 4 months in an attempt to understand what was really going on. He wrote up his discoveries in a book entitled ''Experiments upon Vegetables''. Ingenhousz took green plants and immersed them in water inside a transparent tank. He observed many bubbles rising from the surface of the leaves whenever the plants were exposed to light. Ingenhousz collected the gas that was given off by the plants and performed several different tests in attempt to determine what the gas was. The test that finally revealed the identity of the gas was placing a smouldering taper into the gas sample and having it relight. This test proved it was oxygen, or, as Joseph Priestley had called it, 'de- phlogisticated air'.
In 1932, Robert Emerson and his student, William Arnold, used a repetitive flash technique to precisely measure small quantities of oxygen evolved by chlorophyll in the algae ''Chlorella''. Their experiment proved the existence of a photosynthetic unit. Gaffron and Wohl later interpreted the experiment and realized that the light absorbed by the photosynthetic unit was transferred. This reaction occurs at the reaction center of Photosystem II and takes place in cyanobacteria, algae and green plants.
Photosystem II
Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem ...
is the photosystem that generates the two electrons that will eventually reduce NADP+ in ferredoxin-NADP-reductase. Photosystem II is present on the thylakoid membranes inside chloroplasts, the site of photosynthesis in green plants. The structure of Photosystem II is remarkably similar to the bacterial reaction center, and it is theorized that they share a common ancestor.
The core of Photosystem II consists of two subunits referred to as D1 and D2. These two subunits are similar to the L and M subunits present in the bacterial reaction center. Photosystem II differs from the bacterial reaction center in that it has many additional subunits that bind additional chlorophylls to increase efficiency. The overall reaction catalyzed by Photosystem II is:
:2Q + 2H2O + ''hν'' → O2 + 2QH2
Q represents the oxidized form of plastoquinone while QH2 represents its reduced form. This process of reducing quinone is comparable to that which takes place in the bacterial reaction center. Photosystem II obtains electrons by oxidizing water in a process called photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
. Molecular oxygen is a byproduct of this process, and it is this reaction that supplies the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
with oxygen. The fact that the oxygen from green plants originated from water was first deduced by the Canadian-born American biochemist Martin David Kamen. He used a stable isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are no ...
of oxygen, 18O, to trace the path of the oxygen from water to gaseous molecular oxygen. This reaction is catalyzed by a reactive center in Photosystem II containing four manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
.
The reaction begins with the excitation of a pair of chlorophyll molecules similar to those in the bacterial reaction center. Due to the presence of chlorophyll ''a'', as opposed to bacteriochlorophyll
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacte ...
, Photosystem II absorbs light at a shorter wavelength. The pair of chlorophyll molecules at the reaction center are often referred to as P680. When the photon has been absorbed, the resulting high-energy electron is transferred to a nearby pheophytin molecule. This is above and to the right of the pair on the diagram and is coloured grey. The electron travels from the pheophytin molecule through two plastoquinone molecules, the first tightly bound, the second loosely bound. The tightly bound molecule is shown above the pheophytin molecule and is colored red. The loosely bound molecule is to the left of this and is also colored red. This flow of electrons is similar to that of the bacterial reaction center. Two electrons are required to fully reduce the loosely bound plastoquinone molecule to QH2 as well as the uptake of two protons.
The difference between Photosystem II and the bacterial reaction center is the source of the electron that neutralizes the pair of chlorophyll ''a'' molecules. In the bacterial reaction center, the electron is obtained from a reduced compound haem group in a cytochrome subunit or from a water-soluble cytochrome-c protein.
Every time the P680 absorbs a photon, it gives off an electron to pheophytin, gaining a positive charge. After this photoinduced charge separation
Photoinduced charge separation is the process of an electron in an atom or molecule, being excited to a higher energy level by the absorption of a photon and then leaving the atom or molecule to free space, or to a nearby electron acceptor.
Ruthe ...
, P680+ is a very strong oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
of high energy. It passes its energy to water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
molecules that are bound at the manganese center directly below the pair and extracts an electron from them. This center, below and to the left of the pair in the diagram, contains four manganese ions, a calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
ion, a chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ion, and a tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
residue. Manganese is adept at these reactions because it is capable of existing in four oxidation states: Mn2+, Mn3+, Mn4+ and Mn5+. Manganese also forms strong bonds with oxygen-containing molecules such as water. The process of oxidizing two molecules of water to form an oxygen molecule requires four electrons. The water molecules that are oxidized in the manganese center are the source of the electrons that reduce the two molecules of Q to QH2. To date, this water splitting catalytic center has not been reproduced by any man-made catalyst.
Photosystem I
After the electron has left Photosystem II it is transferred to a cytochrome b6f complex and then to plastocyanin, a bluecopper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
protein and electron carrier. The plastocyanin complex carries the electron that will neutralize the pair in the next reaction center, Photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the Light-dependent reactions, photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane ...
.
As with Photosystem II and the bacterial reaction center, a pair of chlorophyll ''a'' molecules initiates photoinduced charge separation. This pair is referred to as P700
P700, or photosystem I primary donor, is a molecular dimer of chlorophyll ''a'' associated with the reaction-center of photosystem I in plants, algae, and cyanobacteria.
Etymology
Its name is derived from the word “pigment” (P) and the ...
, where 700 is a reference to the wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
at which the chlorophyll molecules absorb light maximally. The P700 lies in the center of the protein. Once photoinduced charge separation has been initiated, the electron travels down a pathway through a chlorophyll α molecule situated directly above the P700, through a quinone molecule situated directly above that, through three 4Fe-4S clusters, and finally to an interchangeable ferredoxin complex. Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
is a soluble protein containing a 2Fe-2S cluster coordinated by four cysteine residues. The positive charge on the high-energy P700+ is neutralized by the transfer of an electron from plastocyanin, which receives energy eventually used to convert QH2 back to Q. Thus the overall reaction catalyzed by Photosystem I is:
:Pc(Cu+) + Fd x/small> + ''hν'' → Pc(Cu2+) + Fd ed/small>
The cooperation between Photosystems I and II creates an electron and proton flow from H2O to NADP+, producing NADPH needed for glucose synthesis. This pathway is called the ' Z-scheme' because the redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
diagram from H2O to NADP+ via P680 and P700 resembles the letter Z.
See also
*Dioxygen in biological reactions
Dioxygen () plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis (light-driven oxidation and splitting) of water during photosynthesis in cyanobacteria, green algae, and ...
(oxygen in biological processes)
*Light-harvesting complex
In biology, a light-harvesting complex or LHC is an aggregate consisting of proteins bound with chromophores (chlorophylls and carotenoids) that play a key role in photosynthesis. LHCs are arrayed around photosynthetic reaction centers in both pl ...
*Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
*Photosystem
Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems ...
*Phycobilisome
Phycobilisomes are light-harvesting antennae that transmit the energy of harvested photons to photosystem II and photosystem I in cyanobacteria and in the chloroplasts of red algae and glaucophytes. They were lost during the evolution of the ...
* Photosynthetic reaction center protein family
References
External links
* {{Multienzyme complexes Light reactions Photosynthesis Integral membrane proteins