|
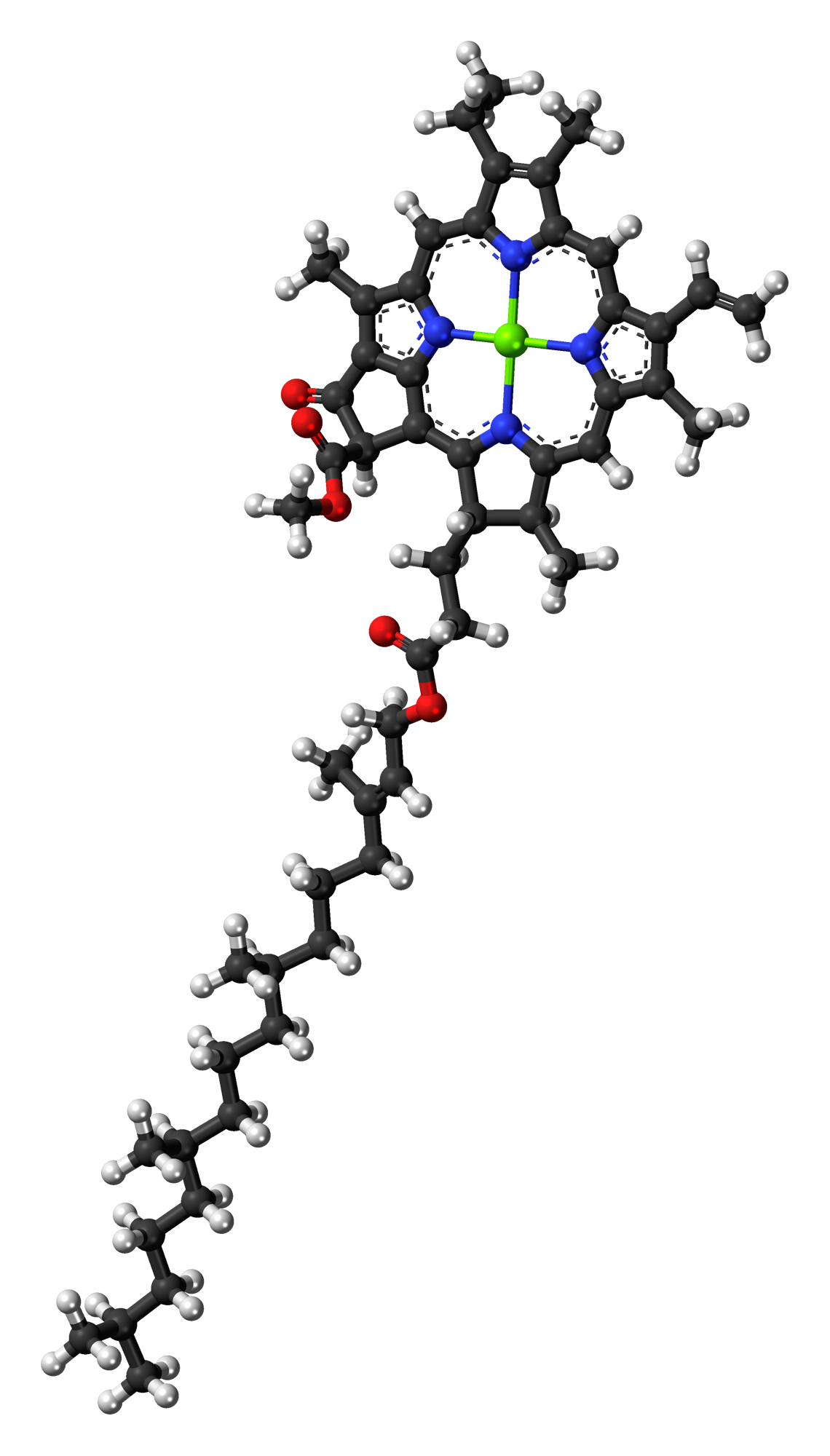
P680
P680, or photosystem II primary donor, is the reaction-center chlorophyll ''a'' molecular dimer associated with photosystem II in plants, algae, and cyanobacteria, and central to oxygenic photosynthesis. Etymology Its name is derived from the word “pigment” (P) and the presence of a major bleaching band centered around 680-685 nm in the flash-induced absorbance difference spectra of P680/ P680+•.Shigeru Itoh, S; Iwaki, M; Tomo, T; Satoh, K (1996). Dibromothymoquinone (DBMIB) replaces the function of QA at 77 K in the isolated photosystem II reaction center (Dl-D2-cytochrome 6559) complex: Difference spectrum of the P680+ (DBMIB") state. Plant Cell Physiol. 37(6): 833-839. Components The structure of P680 consists of a hetero dimer of two distinct chlorophyll molecules, referred to as P and P. This “special pair” forms an excitonic dimer that functions as a single unit, excited by light energy as if they were a single molecule. Action and function Excitat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pheophytin
Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of Photosystem II (PS II) in plants, and the type II photosynthetic reaction center (RC P870) found in purple bacteria. In both PS II and RC P870, light drives electrons from the reaction center through pheophytin, which then passes the electrons to a quinone (QA) in RC P870 and RC P680. The overall mechanisms, roles, and purposes of the pheophytin molecules in the two transport chains are analogous to each other. Structure In biochemical terms, pheophytin is a chlorophyll molecule lacking a central Mg2+ ion. It can be produced from chlorophyll by treatment with a weak acid, producing a dark bluish waxy pigment. The probable etymology comes from this description, with ''pheo'' meaning ''dusky'' and ''phyt'' meaning ''vegetation''. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll A
} Chlorophyll ''a'' is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll ''a'' also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located. Distribution of chlorophyll ''a'' Chlorophyll ''a'' is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chloroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
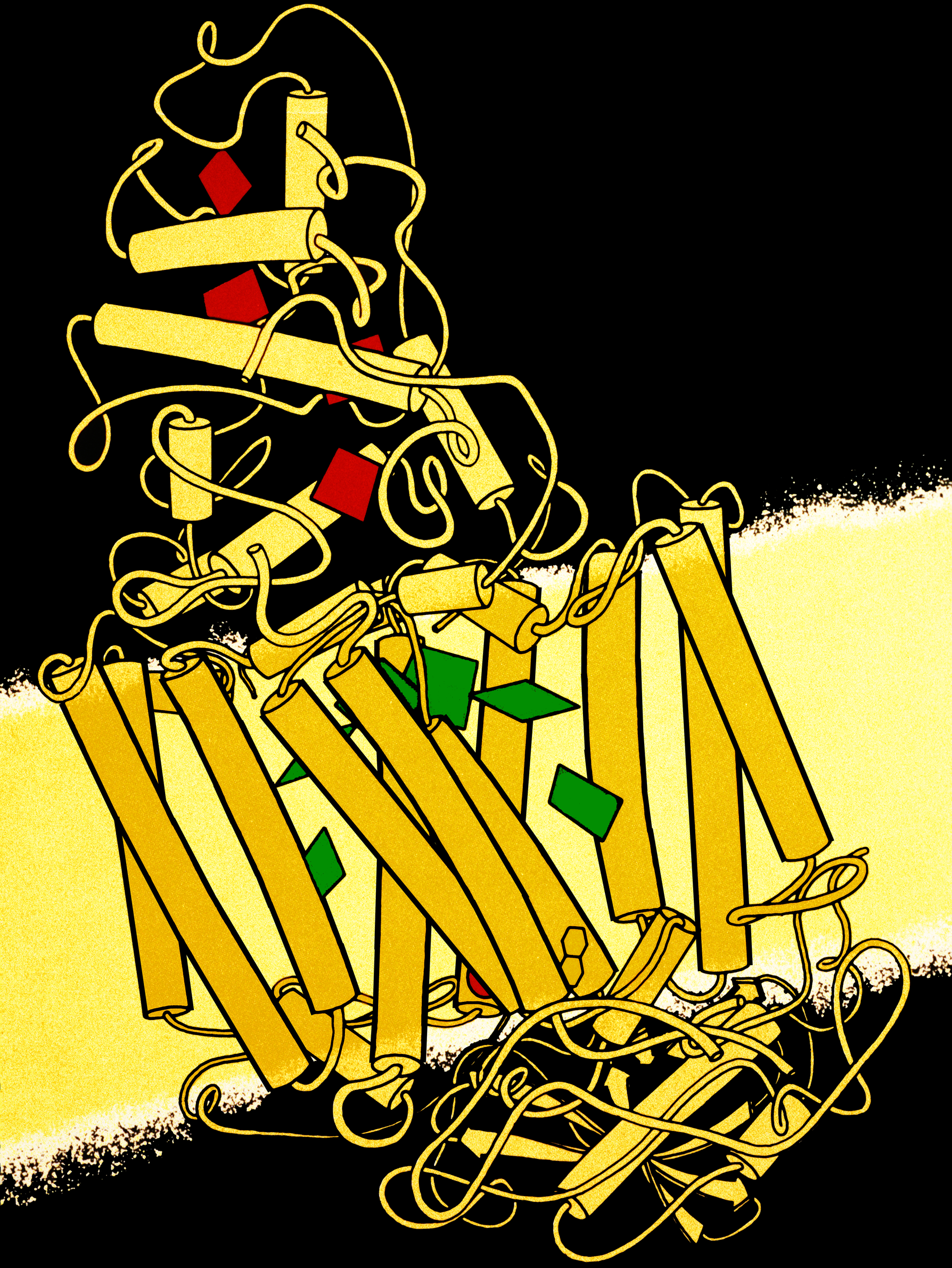
Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen. By replenishing lost electrons with electrons from the splitting of water, photosystem II provides the electrons for all of photosynthesis to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase to generate ATP. The energized electrons transferred to plastoquinone are ultimately used to reduce to NADPH or are used in non-cyclic electron flow. DCMU is a chemical often used in laboratory se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthetic Reaction Centre
A photosynthetic reaction center is a complex of several proteins, biological pigments, and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The Thermodynamic free energy, free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen. By replenishing lost electrons with electrons from the splitting of water, photosystem II provides the electrons for all of photosynthesis to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase to generate ATP. The energized electrons transferred to plastoquinone are ultimately used to reduce to NADPH or are used in non-cyclic electron flow. DCMU is a chemical often used in laboratory se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. ''Photosynthesis'' usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth. Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P700
P700, or photosystem I primary donor, is a molecular dimer of chlorophyll ''a'' associated with the reaction-center of photosystem I in plants, algae, and cyanobacteria. Etymology Its name is derived from the word “pigment” (P) and the presence of a major bleaching band centered around 695-700 nm in the flash-induced absorbance difference spectra of P700/ P700+•. Components The structure of P700 consists of a heterodimer with two distinct chlorophyll molecules, most notably chlorophyll ''a'' and chlorophyll ''a''’, giving it an additional name of “special pair”. Inevitably, however, the special pair of P700 behaves as if it were just one unit. This species is vital due to its ability to absorb light energy with a wavelength approximately between 430 nm-700 nm, and transfer high-energy electrons to a series of acceptors that are situated near it, like ''Fe-S complex, Ferridoxyn(FD), which have a higher redox potential i.e. greater affinity to electron' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ..., an excited state of a system (such as an atom, molecule or Atomic nucleus, nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: Spontaneous emission, spontaneous or stimulated emission, induced emission of a quantum of energy (such as a photon or a phonon) usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiant Energy
In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic radiation, electromagnetic and gravitational radiation. As energy, its SI unit is the joule (J). The quantity of radiant energy may be calculated by Integral, integrating radiant flux (or radiant flux, power) with respect to time. The symbol ''Q''e is often used throughout literature to denote radiant energy ("e" for "energetic", to avoid confusion with photometric quantities). In branches of physics other than radiometry, electromagnetic energy is referred to using ''E'' or ''W''. The term is used particularly when electromagnetic radiation is emitted by a source into the surrounding environment. This radiation may be visible or invisible to the human eye. Terminology use and history The term "radiant energy" is most commonly used in the fields of radiometry, solar energy, heating and lighting, but is also sometimes used in other fields (such as telecommunications). In modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can move no faster than the speed of light measured in vacuum. The photon belongs to the class of boson particles. As with other elementary particles, photons are best explained by quantum mechanics and exhibit wave–particle duality, their behavior featuring properties of both waves and particles. The modern photon concept originated during the first two decades of the 20th century with the work of Albert Einstein, who built upon the research of Max Planck. While Planck was trying to explain how matter and electromagnetic radiation could be in thermal equilibrium with one another, he proposed that the energy stored within a material object should be regarded as composed of an integer number of discrete, equal-sized parts. To explain the pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the atomic nucleus, nucleus, but can also refer to energy levels of nuclei or molecular vibration, vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be Quantization (physics), quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |