Rare-earth-element on:
[Wikipedia]
[Google]
[Amazon]
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the
 As seen in the chart, rare-earth elements are found on Earth at similar concentrations to many common transition metals. The most abundant rare-earth element is
As seen in the chart, rare-earth elements are found on Earth at similar concentrations to many common transition metals. The most abundant rare-earth element is
"Rare earth elements plentiful in ocean sediments"
''ScienceNews'', 3 July 2011. Via Kurt Brouwer'
Fundmastery Blog
, ''MarketWatch'', 2011-07-05. Retrieved 2011-07-05. The global demand for rare-earth elements (REEs) is expected to increase more than fivefold by 2030.
 Until 1948, most of the world's rare earths were sourced from placer deposit, placer sand deposits in India and Brazil. In the 1950s, South Africa was the world's rare earth source, from a monazite-rich reef at the Steenkampskraal mine in Western Cape province. From the 1960s until the 1980s, the Mountain Pass rare earth mine in California made the United States the leading producer. Today, the Indian and South African deposits still produce some rare-earth concentrates, but they were dwarfed by the scale of Chinese production. In 2017, China produced 81% of the world's rare-earth supply, mostly in Inner Mongolia,China's Rare Earth Dominance
Until 1948, most of the world's rare earths were sourced from placer deposit, placer sand deposits in India and Brazil. In the 1950s, South Africa was the world's rare earth source, from a monazite-rich reef at the Steenkampskraal mine in Western Cape province. From the 1960s until the 1980s, the Mountain Pass rare earth mine in California made the United States the leading producer. Today, the Indian and South African deposits still produce some rare-earth concentrates, but they were dwarfed by the scale of Chinese production. In 2017, China produced 81% of the world's rare-earth supply, mostly in Inner Mongolia,China's Rare Earth Dominance
Wikinvest. Retrieved on 11 Aug 2010. although it had only 36.7% of reserves. In 2018, Australia was the world's second largest producer, and the only other major producer, with 15% of world production. All of the world's heavy rare earths (such as dysprosium) come from Chinese rare-earth sources such as the polymetallic Bayan Obo deposit. The Browns Range mine, located 160 km south east of Halls Creek in northern Western Australia, was under development in 2018 and is positioned to become the first significant dysprosium producer outside of China. REE is increasing in demand due to the fact that they are essential for new and innovative technology that is being created. These new products that need REEs to be produced are high-technology equipment such as smart phones, digital cameras, computer parts, semiconductors, etc. In addition, these elements are more prevalent in the following industries: renewable energy technology, military equipment, glass making, and metallurgy. Increased demand has strained supply, and there is growing concern that the world may soon face a shortage of the rare earths. In 2009, future worldwide demand for rare-earth elements was expected to exceed supply by 40,000 metric tons annually unless major new sources are developed."As hybrid cars gobble rare metals, shortage looms"
. Reuters. August 31, 2009. Retrieved Aug 31, 2009. In 2013, it was stated that the demand for REEs would increase due to the dependence of the EU on these elements, the fact that rare-earth elements cannot be substituted by other elements and that REEs have a low recycling rate. Due to the increased demand and low supply, future prices are expected to increase and there is a chance that countries other than China will open REE mines. In 2023, there were over a hundred ongoing mining projects, with many options outside of China. As a result of the increased demand and tightening restrictions on exports of the metals from China, some countries are stockpiling rare-earth resources. Searches for alternative sources in Australia, Brazil, Canada, South Africa, Tanzania, Greenland, and the United States are ongoing. Mines in these countries were closed when China undercut world prices in the 1990s, and it will take a few years to restart production as there are many barriers to entry.
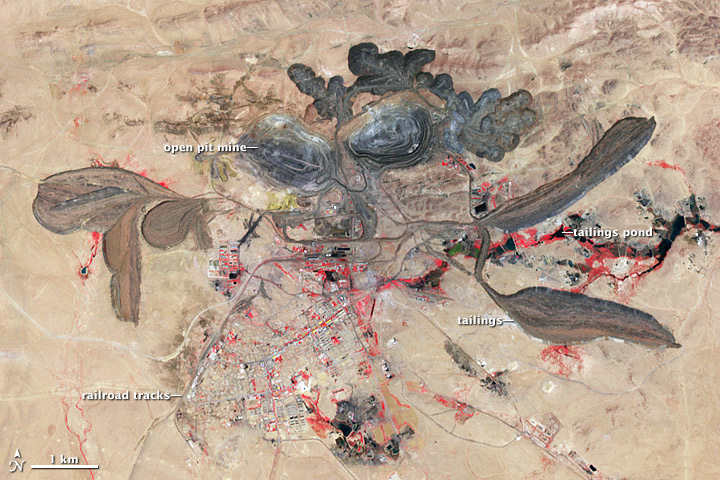 Mining, refining, and recycling of rare earths have serious environmental consequences if not properly managed. Low-level radioactive tailings resulting from the occurrence of
Mining, refining, and recycling of rare earths have serious environmental consequences if not properly managed. Low-level radioactive tailings resulting from the occurrence of
"Malaysia Rare Earths in Largest Would-Be Refinery Incite Protest"
, ''Bloomberg L.P., Bloomberg Markets Magazine'', May 31, 2011 5:00 PM ET. Construction of the facility has been halted until an independent United Nations IAEA panel investigation is completed, which is expected by the end of June 2011. #Production, New restrictions were announced by the Malaysian government in late June. An IAEA panel investigation was completed and no construction has been halted. Lynas is on budget and on schedule to start producing in 2011. The IAEA concluded in a report issued in June 2011 that it did not find any instance of "any non-compliance with international radiation safety standards" in the project. If the proper safety standards are followed, REE mining is relatively low impact. Molycorp (before going bankrupt) often exceeded environmental regulations to improve its public image. In Greenland, there is a significant dispute on whether to start a new rare-earth mine in Kvanefjeld due to environmental concerns.
 China has officially cited resource depletion and environmental concerns as the reasons for a nationwide crackdown on its rare-earth mineral production sector. Non-environmental motives have also been imputed to China's rare-earth policy. In 2010, according to ''The Economist'', "Slashing their exports of rare-earth metals ... is all about moving Chinese manufacturers up the supply chain, so they can sell valuable finished goods to the world rather than lowly raw materials."
China currently has an effective monopoly on the world's REE Value Chain. (All of the refineries and processing plants that transform the raw ore into valuable elements.) In the words of Deng Xiaoping, a Chinese politician from the late 1970s to the late 1980s, "The Middle East has oil; we have rare earths ... it is of extremely important strategic significance; we must be sure to handle the rare earth issue properly and make the fullest use of our country's advantage in rare-earth resources."
One possible example of market control is the division of General Motors that deals with miniaturized magnet research, which shut down its US office and moved its entire staff to China in 2006 China's export quota only applies to the metal but not products made from these metals such as magnets.
It was reported, but officially denied, that China instituted an economic sanctions, export ban on shipments of rare-earth oxides, but not alloys, to Japan on 22 September 2010, in response to 2010 Senkaku boat collision incident, the detainment of a Chinese fishing boat captain by the Japanese Coast Guard. On September 2, 2010, a few days before the fishing boat incident, ''The Economist'' reported that "China ... in July announced the latest in a series of annual export reductions, this time by 40% to precisely 30,258 tonnes."
The United States Department of Energy in its 2010 Critical Materials Strategy report identified dysprosium as the element that was most critical in terms of import reliance.
A 2011 report "China's Rare-Earth Industry", issued by the US Geological Survey and US Department of the Interior, outlines industry trends within China and examines national policies that may guide the future of the country's production. The report notes that China's lead in the production of rare-earth minerals has accelerated over the past two decades. In 1990, China accounted for only 27% of such minerals. In 2009, world production was 132,000 metric tons; China produced 129,000 of those tons. According to the report, recent patterns suggest that China will slow the export of such materials to the world: "Owing to the increase in domestic demand, the Government has gradually reduced the export quota during the past several years."
In 2006, China allowed 47 domestic rare-earth producers and traders and 12 Sino-foreign rare-earth producers to export. Controls have since tightened annually; by 2011, only 22 domestic rare-earth producers and traders and 9 Sino-foreign rare-earth producers were authorized. The government's future policies will likely keep in place strict controls: "According to China's draft rare-earth development plan, annual rare-earth production may be limited to between 130,000 and 140,000 [metric tons] during the period from 2009 to 2015. The export quota for rare-earth products may be about 35,000 [metric tons] and the Government may allow 20 domestic rare-earth producers and traders to export rare earths."
The United States Geological Survey was actively surveying southern Afghanistan for rare-earth deposits under the protection of United States military forces. Since 2009 the USGS has conducted remote sensing surveys as well as fieldwork to verify Soviet claims that volcanic rocks containing rare-earth metals exist in Helmand Province near the village of Khanashin. The USGS study team has located a sizable area of rocks in the center of an extinct volcano containing light rare-earth elements including cerium and neodymium. It has mapped 1.3 million metric tons of desirable rock, or about ten years of supply at current demand levels. The Pentagon has estimated its value at about $7.4 billion.
It has been argued that the geopolitical importance of rare earths has been exaggerated in the literature on the geopolitics of renewable energy, underestimating the power of economic incentives for expanded production. This especially concerns neodymium. Due to its role in permanent magnets used for wind turbines, it has been argued that neodymium will be one of the main objects of geopolitical competition in a world running on renewable energy. But this perspective has been criticized for failing to recognize that most wind turbines have gears and do not use permanent magnets.
China has officially cited resource depletion and environmental concerns as the reasons for a nationwide crackdown on its rare-earth mineral production sector. Non-environmental motives have also been imputed to China's rare-earth policy. In 2010, according to ''The Economist'', "Slashing their exports of rare-earth metals ... is all about moving Chinese manufacturers up the supply chain, so they can sell valuable finished goods to the world rather than lowly raw materials."
China currently has an effective monopoly on the world's REE Value Chain. (All of the refineries and processing plants that transform the raw ore into valuable elements.) In the words of Deng Xiaoping, a Chinese politician from the late 1970s to the late 1980s, "The Middle East has oil; we have rare earths ... it is of extremely important strategic significance; we must be sure to handle the rare earth issue properly and make the fullest use of our country's advantage in rare-earth resources."
One possible example of market control is the division of General Motors that deals with miniaturized magnet research, which shut down its US office and moved its entire staff to China in 2006 China's export quota only applies to the metal but not products made from these metals such as magnets.
It was reported, but officially denied, that China instituted an economic sanctions, export ban on shipments of rare-earth oxides, but not alloys, to Japan on 22 September 2010, in response to 2010 Senkaku boat collision incident, the detainment of a Chinese fishing boat captain by the Japanese Coast Guard. On September 2, 2010, a few days before the fishing boat incident, ''The Economist'' reported that "China ... in July announced the latest in a series of annual export reductions, this time by 40% to precisely 30,258 tonnes."
The United States Department of Energy in its 2010 Critical Materials Strategy report identified dysprosium as the element that was most critical in terms of import reliance.
A 2011 report "China's Rare-Earth Industry", issued by the US Geological Survey and US Department of the Interior, outlines industry trends within China and examines national policies that may guide the future of the country's production. The report notes that China's lead in the production of rare-earth minerals has accelerated over the past two decades. In 1990, China accounted for only 27% of such minerals. In 2009, world production was 132,000 metric tons; China produced 129,000 of those tons. According to the report, recent patterns suggest that China will slow the export of such materials to the world: "Owing to the increase in domestic demand, the Government has gradually reduced the export quota during the past several years."
In 2006, China allowed 47 domestic rare-earth producers and traders and 12 Sino-foreign rare-earth producers to export. Controls have since tightened annually; by 2011, only 22 domestic rare-earth producers and traders and 9 Sino-foreign rare-earth producers were authorized. The government's future policies will likely keep in place strict controls: "According to China's draft rare-earth development plan, annual rare-earth production may be limited to between 130,000 and 140,000 [metric tons] during the period from 2009 to 2015. The export quota for rare-earth products may be about 35,000 [metric tons] and the Government may allow 20 domestic rare-earth producers and traders to export rare earths."
The United States Geological Survey was actively surveying southern Afghanistan for rare-earth deposits under the protection of United States military forces. Since 2009 the USGS has conducted remote sensing surveys as well as fieldwork to verify Soviet claims that volcanic rocks containing rare-earth metals exist in Helmand Province near the village of Khanashin. The USGS study team has located a sizable area of rocks in the center of an extinct volcano containing light rare-earth elements including cerium and neodymium. It has mapped 1.3 million metric tons of desirable rock, or about ten years of supply at current demand levels. The Pentagon has estimated its value at about $7.4 billion.
It has been argued that the geopolitical importance of rare earths has been exaggerated in the literature on the geopolitics of renewable energy, underestimating the power of economic incentives for expanded production. This especially concerns neodymium. Due to its role in permanent magnets used for wind turbines, it has been argued that neodymium will be one of the main objects of geopolitical competition in a world running on renewable energy. But this perspective has been criticized for failing to recognize that most wind turbines have gears and do not use permanent magnets.
lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s or lanthanoids (although scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
and yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals
upright=1.2, Crystals of lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals is a controversial and ambiguous term for metallic elements with relatively h ...
. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes.
The term "rare-earth" is a misnomer
A misnomer is a name that is incorrectly or unsuitably applied. Misnomers often arise because something was named long before its correct nature was known, or because an earlier form of something has been replaced by a later form to which the nam ...
because they are not actually scarce, but historically it took a long time to isolate these elements.
They are relatively plentiful in the entire Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
(cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
being the 25th-most-abundant element at 68 parts per million, more abundant than copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths.
Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically including metals, concentrated above background levels, and that is economically viable to mine and process. The grade of ore refers to the concentration ...
deposits as the lanthanides and exhibit similar chemical properties, but have different electrical and magnetic properties
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, m ...
.
These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides and ignite spontaneously at a temperature of . These elements and their compounds have no biological function other than in several specialized enzymes, such as in lanthanide-dependent methanol dehydrogenases in bacteria. The water-soluble compounds are mildly to moderately toxic, but the insoluble ones are not. All isotopes of promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
are radioactive, and it does not occur naturally in the earth's crust, except for a trace amount generated by spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
. They are often found in mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s with thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, and less commonly uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
.
Because of their geochemical
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the ...
properties, rare-earth elements are typically dispersed and not often found concentrated in rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s. Consequently, economically exploitable ore deposits
Ore is natural rock or sediment that contains one or more valuable minerals, typically including metals, concentrated above background levels, and that is economically viable to mine and process. The grade of ore refers to the concentration o ...
are sparse. The first rare-earth mineral discovered (1787) was gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending o ...
, a black mineral composed of cerium, yttrium, iron, silicon, and other elements. This mineral was extracted from a mine in the village of Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is the ...
in Sweden
Sweden, formally the Kingdom of Sweden, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. It borders Norway to the west and north, and Finland to the east. At , Sweden is the largest Nordic count ...
. Four of the rare-earth elements bear names derived from this single location.
Elements
A table listing the 17 rare-earth elements, theiratomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
and symbol, the etymology of their names, and their main uses (see also Applications of lanthanides) is provided here. Some of the rare-earth elements are named after the scientists who discovered them, or elucidated their elemental properties, and some after the geographical locations where discovered.
A mnemonic for the names of the sixth-row elements in order is "Lately college parties never produce sexy European girls that drink heavily even though you look".
Discovery and early history
Rare earths were mainly discovered as components of minerals. The term "rare" refers to these rarely found minerals and "earth" comes from an old name for oxides, the chemical form for these elements in the mineral. The adjective "rare" may also mean strange or extraordinary. In 1787, a mineral discovered by LieutenantCarl Axel Arrhenius
Carl Axel Arrhenius (29 March 1757 – 20 November 1824) was a Swedish military officer, amateur geologist, and chemist. He is best known for his discovery of the mineral ytterbite (later called gadolinite) in 1787.
The discovery of ytterbit ...
at a quarry in the village of Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is the ...
, Sweden, reached Johan Gadolin
Johan Gadolin (5 June 176015 August 1852) was a Finnish chemist, physicist and mineralogist. Gadolin discovered a " new earth" containing the first rare-earth compound yttrium, which was later determined to be a chemical element. He is also con ...
, a Royal Academy of Turku
The Royal Academy of Turku or the Royal Academy of Åbo was the first university in Finland, and the only Finnish university that was founded when the country still was a part of Sweden. It was founded in 1640. In 1809, after Finland became a ...
professor, and his analysis yielded an unknown oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
which he called yttria
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance.
The thermal conductivity of yttrium oxide is 27 W/(m·K).
Applications Phosphors
Yttrium oxide is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that ...
.
Anders Gustav Ekeberg isolated beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
from the gadolinite but failed to recognize other elements in the ore. After this discovery in 1794, a mineral from Bastnäs
Bastnäs ( or ) is an ore field near Riddarhyttan, Västmanland, Sweden. The mines in Bastnäs were earliest mentioned in 1692. Iron, copper and rare-earth elements were extracted from the mines and 4,500 tons of cerium was produced between 1875 a ...
near Riddarhyttan
Riddarhyttan is a urban areas of Sweden, locality in Skinnskatteberg Municipality, Västmanland County, Sweden, with 431 inhabitants in 2010. It has an old iron mining tradition, which can be followed back to the last centuries before Christ. The ...
, Sweden, which was believed to be an iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
–tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
mineral, was re-examined by Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be one of the founders of modern chemistry. Berzelius became a memb ...
and Wilhelm Hisinger
Wilhelm Hisinger (23 December 1766 – 28 June 1852) was a Swedish physicist and chemist who in 1807, working in coordination with Jöns Jakob Berzelius, noted that in electrolysis any given substance always went to the same pole, and that substan ...
. In 1803, they obtained a white oxide and called it ceria
Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial produc ...
. Martin Heinrich Klaproth
Martin Heinrich Klaproth (1 December 1743 – 1 January 1817) was a German chemist. He trained and worked for much of his life as an apothecary, moving in later life to the university. His shop became the second-largest apothecary in Berlin, and ...
independently discovered the same oxide and called it ''ochroia''. It took another 30 years for researchers to determine that other elements were contained in the two ores ceria and yttria. The similarity of the rare-earth metals' chemical properties made their separation difficult.
In 1839, Carl Gustav Mosander
Carl Gustaf Mosander (10 September 1797 – 15 October 1858) was a Swedish chemist. He discovered the rare earth elements lanthanum, erbium and terbium.
Early life and education
Born in Kalmar, Mosander attended school there until he move ...
, an assistant of Berzelius, separated ceria by heating the nitrate and dissolving the product in nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
. He called the oxide of the soluble salt ''lanthana''. It took him three more years to separate the lanthana further into ''didymia'' and pure lanthana. Didymia, although not further separable by Mosander's techniques, was in fact still a mixture of oxides.
In 1842, Mosander separated the yttria into three oxides: pure yttria, terbia, and erbia. All the names are derived from the town name "Ytterby". The earth giving pink salts he called ''terbium''. The one that yielded yellow peroxide he called ''erbium''.
By then the number of known rare-earth elements had reached six: yttrium, cerium, lanthanum, didymium, erbium, and terbium.
Nils Johan Berlin
Nils Johan Berlin (Nils Johannes Berlin) (18 February 1812 – 27 December 1891) was a Sweden, Swedish chemist and physician, who held various professorships at the University of Lund from 1843 to 1864. Berlin was the first chemist who took the i ...
and Marc Delafontaine
Marc Delafontaine (March 31, 1837/1838, Céligny, Switzerland–1911) was a Switzerland, Swiss chemist and spectroscopist who was involved in discovering and investigating some of the rare earth elements.
Career
Delafontaine studied with Jean C ...
tried also to separate the crude yttria and found the same substances that Mosander obtained. In 1860, Berlin named the substance giving pink salts ''erbium''. Delafontaine named the substance with the yellow peroxide, ''terbium''. This confusion led to several false claims of new elements, such as the ''mosandrium'' of J. Lawrence Smith, or the ''philippium'' and '' decipium'' of Delafontaine. Due to the difficulty in separating the metals, and determining the separation is complete, the total number of false discoveries was dozens, with some putting the total number of discoveries at over a hundred.
Spectroscopic identification
There were no further discoveries for 30 years, and the elementdidymium
Didymium () is a mixture of the elements praseodymium and neodymium. It is used in safety glasses for glassblowing and blacksmithing and filter lenses for flame testing, especially with a gas (propane)-powered forge, where it provides a filt ...
was listed in the periodic table of elements with a molecular mass of 138. In 1879, Delafontaine used the new physical process of optical flame spectroscopy and found several new spectral lines in didymia. Also in 1879, Paul Émile Lecoq de Boisbaudran
Paul may refer to:
People
* Paul (given name), a given name, including a list of people
* Paul (surname), a list of people
* Paul the Apostle, an apostle who wrote many of the books of the New Testament
* Ray Hildebrand, half of the singing duo ...
isolated the new element ''samarium
Samarium is a chemical element; it has symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of s ...
'' from the mineral samarskite
Samarskite is a radioactive rare earth mineral series which includes samarskite-(Y), with the chemical formula and samarskite-(Yb), with the chemical formula . The formula for samarskite-(Y) is also given as .
Samarskite crystallizes in the ort ...
.
In 1886, the samaria earth was further separated by Lecoq de Boisbaudran. A similar result was obtained by Jean Charles Galissard de Marignac
Jean Charles Galissard de Marignac (24 April 1817 – 15 April 1894) was a Swiss chemist whose work with atomic weights suggested the possibility of isotopes and the packing fraction of nuclei. His study of the rare earth elements led to ...
by direct isolation from samarskite. They named the element ''gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
'' after Johan Gadolin
Johan Gadolin (5 June 176015 August 1852) was a Finnish chemist, physicist and mineralogist. Gadolin discovered a " new earth" containing the first rare-earth compound yttrium, which was later determined to be a chemical element. He is also con ...
, and its oxide was named " gadolinia".
Further spectroscopic analysis between 1886 and 1901 of samaria, yttria, and samarskite by William Crookes
Sir William Crookes (; 17 June 1832 – 4 April 1919) was an English chemist and physicist who attended the Royal College of Chemistry, now part of Imperial College London, and worked on spectroscopy. He was a pioneer of vacuum tubes, inventing ...
, Lecoq de Boisbaudran and Eugène-Anatole Demarçay
Eugène-Anatole Demarçay (1 January 1852 – 5 March 1903) was a French chemist who designed an apparatus to produce a spark using an induction coil and used it to generate the spectra of rare earth elements which he examined using spectroscop ...
yielded several new spectral line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency ...
s that indicated the existence of an unknown element. In 1901, the fractional crystallization Fractional crystallization may refer to:
* Fractional crystallization (chemistry), a process to separate different solutes from a solution
* Fractional crystallization (geology)
Fractional crystallization, or crystal fractionation, is one of the ...
of the oxides yielded ''europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
''.
In 1839, the third source for rare earths became available. This is a mineral similar to gadolinite called ''uranotantalum'', now called "samarskite
Samarskite is a radioactive rare earth mineral series which includes samarskite-(Y), with the chemical formula and samarskite-(Yb), with the chemical formula . The formula for samarskite-(Y) is also given as .
Samarskite crystallizes in the ort ...
", an oxide of a mixture of elements such as yttrium, ytterbium, iron, uranium, thorium, calcium, niobium, and tantalum. This mineral from Miass
Miass (, ) is a city in Chelyabinsk Oblast, Russia, located west of Chelyabinsk, on the eastern slope of the Southern Ural Mountains, on the bank of the river Miass. Population:
Name
The name Miass is taken from the Bashkirs (), the indige ...
in the southern Ural Mountains
The Ural Mountains ( ),; , ; , or simply the Urals, are a mountain range in Eurasia that runs north–south mostly through Russia, from the coast of the Arctic Ocean to the river Ural (river), Ural and northwestern Kazakhstan.
was documented by Gustav Rose
Prof Gustavus ("Gustav") Rose Royal Society of London, FRSFor HFRSE (18 March 1798 – 15 July 1873) was a German mineralogist who was a native of Berlin. He was President of the German Geological Society from 1863 to 1873.
Life
He was born in Be ...
. The Russian chemist R. Harmann proposed that a new element he called " ilmenium" should be present in this mineral, but later, Christian Wilhelm Blomstrand
Christian Wilhelm Blomstrand (20 October 1826 – 5 November 1897) was a Swedish mineralogist and chemist. He was a professor at the University of Lund from 1862-1895, where he isolated the element niobium in 1864. He developed an early version o ...
, Galissard de Marignac, and Heinrich Rose
Heinrich Rose (6 August 1795 – 27 January 1864) was a German mineralogist and analytical chemist. He was the brother of the mineralogist Gustav Rose and a son of Valentin Rose.
Rose's early works on phosphorescence were noted in the Quarterly J ...
found only tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
and niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
(columbium
Niobium is a chemical element; it has symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has similar ...
) in it.
The exact number of rare-earth elements that existed was highly unclear, and a maximum number of 25 was estimated. Using X-ray spectra Henry Gwyn Jeffreys Moseley confirmed the atomic theory of Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
and simultaneously developed the theory of atomic numbers for the elements. Moseley found that the exact number of lanthanides had to be 15, revealing a missing element, element 61
Promethium is a chemical element; it has symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust at any given time. Promethium is one of ...
, a radioactive element with a half-life of 18 years.
Using these facts about atomic numbers from X-ray crystallography, Moseley also showed that hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
(element 72) would not be a rare-earth element. Moseley was killed in World War I
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
in 1915, years before hafnium was discovered. Hence, the claim of Georges Urbain
Georges Urbain (12 April 1872 – 5 November 1938) was a French chemist, a professor of the Sorbonne, a member of the Institut de France, and director of the Institute of Chemistry in Paris. Much of his work focused on the rare earths, isolating ...
that he had discovered element 72 was untrue. Hafnium is an element that lies in the periodic table immediately below zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, and hafnium and zirconium have very similar chemical and physical properties.
Sources and purification
In the 1940s, Frank Spedding and others in the United States, during theManhattan Project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada.
From 1942 to 1946, the ...
, developed chemical ion-exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
procedures for separating and purifying rare-earth elements. This method was first applied to the actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s for separating plutonium-239
Plutonium-239 ( or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main iso ...
and neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar Syste ...
from uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
, and the other actinides in the materials produced in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s. Plutonium-239 was very desirable because it is a fissile material
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal chain reaction can only be achieved with fissile material. The predominant neutron energy i ...
.
The principal sources of rare-earth elements are the minerals bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yt ...
(, where R is a mixture of rare-earth elements), monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium ...
(, where X is a mixture of rare-earth elements and sometimes thorium), and loparite
Loparite-(Ce) is a granular, brittle oxide mineral of the perovskite class. It is black to dark grey and may appear grey to white in reflected light on polished thin section with reddish brown internal reflections. It has the chemical formula of . ...
(), and the lateritic
Laterite is a soil type rich in iron and aluminium and is commonly considered to have formed in hot and wet tropical areas. Nearly all laterites are of rusty-red coloration, because of high iron oxide content. They develop by intensive and prolo ...
ion-adsorption clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impuriti ...
s. Despite their high relative abundance, rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s are more difficult to mine and extract than equivalent sources of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s, due in part to their similar chemical properties, making the rare-earth elements relatively expensive. Their industrial use was very limited until efficient separation techniques were developed, such as ion exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
, fractional crystallization, and liquid–liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubility, solubilities in two different Miscibility, immiscible liquids, usually wate ...
in the late 1950s and early 1960s.
Some ilmenite
Ilmenite is a titanium-iron oxide mineral with the idealized formula . It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printi ...
concentrates contain small amounts of scandium and other rare-earth elements, which could be analysed by X-ray fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis ...
(XRF).
Classification
Before the time thation exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
methods and elution
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent: washing of loaded ion-exchange resins to remove captured ions, or eluting proteins or other biopolymers from an el ...
were available, the separation of the rare earths was primarily achieved by repeated precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
or crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
. In those days, the first separation was into two main groups, the cerium earths (lanthanum, cerium, praseodymium, neodymium, and samarium) and the yttrium earths (scandium, yttrium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium).
Europium, gadolinium, and terbium were either considered as a separate group of rare-earth elements (the terbium group), or europium was included in the cerium group, and gadolinium and terbium were included in the yttrium group. In the latter case, the f-block elements are split into half: the first half (La–Eu) form the cerium group, and the second half (Gd–Yb) together with group 3 (Sc, Y, Lu) form the yttrium group.
The reason for this division arose from the difference in solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
of rare-earth double sulfates with sodium and potassium. The sodium double sulfates of the cerium group are poorly soluble, those of the terbium group slightly, and those of the yttrium group are very soluble. Sometimes, the yttrium group was further split into the erbium group (dysprosium, holmium, erbium, and thulium) and the ytterbium group (ytterbium and lutetium), but today the main grouping is between the cerium and the yttrium groups. Today, the rare-earth elements are classified as light or heavy rare-earth elements, rather than in cerium and yttrium groups.
Light versus heavy classification
The classification of rare-earth elements is inconsistent between authors. The most common distinction between rare-earth elements is made byatomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
s. Those with low atomic numbers are referred to as light rare-earth elements (LREE), those with high atomic numbers are the heavy rare-earth elements (HREE), and those that fall in between are typically referred to as the middle rare-earth elements (MREE). Commonly, rare-earth elements with atomic numbers 57 to 61 (lanthanum to promethium) are classified as light and those with atomic numbers 62 and greater are classified as heavy rare-earth elements.
Increasing atomic numbers between light and heavy rare-earth elements and decreasing atomic radii
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
throughout the series causes chemical variations. Europium is exempt of this classification as it has two valence states: Eu and Eu. Yttrium is grouped as a heavy rare-earth element due to chemical similarities. The break between the two groups is sometimes put elsewhere, such as between elements 63 (europium) and 64 (gadolinium). The actual metallic densities of these two groups overlap, with the "light" group having densities from 6.145 (lanthanum) to 7.26 (promethium) or 7.52 (samarium) g/cc, and the "heavy" group from 6.965 (ytterbium) to 9.32 (thulium), as well as including yttrium at 4.47. Europium has a density of 5.24.
Origin
Rare-earth elements, exceptscandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, are heavier than iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
and thus are produced by supernova nucleosynthesis
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
In sufficiently massive stars, the nucleosynthesis by fusion of lighter elements into heavier ones occurs during sequential hydrostatic burning process ...
or by the s-process
The slow neutron-capture process, or ''s''-process, is a series of nuclear reactions, reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynt ...
in asymptotic giant branch
The asymptotic giant branch (AGB) is a region of the Hertzsprung–Russell diagram populated by evolved cool luminous stars. This is a period of stellar evolution undertaken by all low- to intermediate-mass stars (about 0.5 to 8 solar masses) lat ...
stars. In nature, spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
produces trace amounts of radioactive promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
, but most promethium is synthetically produced in nuclear reactors.
Due to their chemical similarity, the concentrations of rare earths in rocks are only slowly changed by geochemical processes, making their proportions useful for geochronology
Geochronology is the science of Chronological dating, determining the age of rock (geology), rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, ...
and dating fossils.
Compounds
Rare-earth elements occur in nature in combination withphosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
(monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium ...
), carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
-fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
(bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yt ...
), and oxygen anions.
In their oxides, most rare-earth elements only have a valence of 3 and form sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides.
Many sesquioxid ...
s (cerium forms ). Five different crystal structures are known, depending on the element and the temperature. The X-phase and the H-phase are only stable above 2000 K. At lower temperatures, there are the hexagonal A-phase, the monoclinic B-phase, and the cubic C-phase, which is the stable form at room temperature for most of the elements. The C-phase was once thought to be in space group ''I''23 (no. 199), but is now known to be in space group ''Ia'' (no. 206).
The structure is similar to that of fluorite or cerium dioxide (in which the cations form a face-centred cubic lattice and the anions sit inside the tetrahedra of cations), except that one-quarter of the anions (oxygen) are missing. The unit cell of these sesquioxides corresponds to eight unit cells of fluorite or cerium dioxide, with 32 cations instead of 4. This is called the bixbyite structure, as it occurs in a mineral of that name ().
Geological distribution
cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
, which is actually the 25th most abundant element in crust (geology), Earth's crust, having 68 parts per million (about as common as copper). The exception is the highly unstable and radioactive promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
"rare earth" is quite scarce. The longest-lived isotope of promethium has a half-life of 17.7 years, so the element exists in nature in only negligible amounts (approximately 572 g in the entire Earth's crust). Promethium is one of the two elements that do not have stable (non-radioactive) isotopes and are followed by (i.e. with higher atomic number) stable elements (the other being technetium).
The rare-earth elements are often found together. During the sequential accretion (geology), accretion of the Earth, the dense rare-earth elements were incorporated into the deeper portions of the planet. Early differentiation of molten material largely incorporated the rare earths into Mantle (geology), mantle rocks. The magnetic moment, high field strength and large ionic radius, ionic radii of rare earths make them incompatible with the crystal lattices of most rock-forming minerals, so REE will undergo strong partitioning into a melt phase if one is present.
REE are chemically very similar and have always been difficult to separate, but the gradual decrease in ionic radius from light REE (LREE) to heavy REE (HREE), called the lanthanide contraction, can produce a broad separation between light and heavy REE. The larger ionic radii of LREE make them generally more incompatible than HREE in rock-forming minerals, and will partition more strongly into a melt phase, while HREE may prefer to remain in the crystalline residue, particularly if it contains HREE-compatible minerals like garnet. The result is that all magma formed from partial melting will always have greater concentrations of LREE than HREE, and individual minerals may be dominated by either HREE or LREE, depending on which range of ionic radii best fits the crystal lattice.
Among the anhydrous rare-earth phosphates, it is the tetragonal mineral xenotime that incorporates yttrium and the HREE, whereas the monoclinic monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium ...
phase incorporates cerium and the LREE preferentially. The smaller size of the HREE allows greater solid solubility in the rock-forming minerals that make up Earth's mantle, and thus yttrium and the HREE show less enrichment in Earth's crust relative to chondrite, chondritic abundance than does cerium and the LREE.
This has economic consequences: large ore bodies of LREE are known around the world and are being exploited. Ore bodies for HREE are more rare, smaller, and less concentrated. Most of the current supply of HREE originates in the "ion-absorption clay" ores of Southern China. Some versions provide concentrates containing about 65% yttrium oxide, with the HREE being present in ratios reflecting the Oddo–Harkins rule: even-numbered REE at abundances of about 5% each, and odd-numbered REE at abundances of about 1% each. Similar compositions are found in xenotime or gadolinite.
Well-known minerals containing yttrium, and other HREE, include gadolinite, xenotime, samarskite
Samarskite is a radioactive rare earth mineral series which includes samarskite-(Y), with the chemical formula and samarskite-(Yb), with the chemical formula . The formula for samarskite-(Y) is also given as .
Samarskite crystallizes in the ort ...
, euxenite, fergusonite, yttrotantalite, yttrotungstite, yttrofluorite (a variety of fluorite), thalenite, and yttrialite. Small amounts occur in zircon, which derives its typical yellow fluorescence from some of the accompanying HREE. The zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
mineral eudialyte, such as is found in southern Greenland, contains small but potentially useful amounts of yttrium. Of the above yttrium minerals, most played a part in providing research quantities of lanthanides during the discovery days. Xenotime is occasionally recovered as a byproduct of heavy-sand processing, but is not as abundant as the similarly recovered monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium ...
(which typically contains a few percent of yttrium). Uranium ores from Ontario have occasionally yielded yttrium as a byproduct.
Well-known minerals containing cerium, and other LREE, include bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yt ...
, monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium ...
, allanite, loparite
Loparite-(Ce) is a granular, brittle oxide mineral of the perovskite class. It is black to dark grey and may appear grey to white in reflected light on polished thin section with reddish brown internal reflections. It has the chemical formula of . ...
, ancylite, parisite, lanthanite, chevkinite, cerite, stillwellite, britholite, fluocerite, and cerianite. Monazite (marine sands from Brazil, India, or Australia; rock from South Africa), bastnäsite (from Mountain Pass rare earth mine, or several localities in China), and loparite
Loparite-(Ce) is a granular, brittle oxide mineral of the perovskite class. It is black to dark grey and may appear grey to white in reflected light on polished thin section with reddish brown internal reflections. It has the chemical formula of . ...
(Kola Peninsula, Russia) have been the principal ores of cerium and the light lanthanides.
Enriched deposits of rare-earth elements at the surface of the Earth, carbonatites and pegmatites, are related to alkaline plutonism, an uncommon kind of magmatism that occurs in tectonic settings where there is rifting or that are near subduction zones. In a rift setting, the alkaline magma is produced by very small degrees of partial melting (<1%) of garnet peridotite in the upper mantle (Earth), upper mantle (200 to 600 km depth). This melt becomes enriched in incompatible elements, like the rare-earth elements, by leaching them out of the crystalline residue. The resultant magma rises as a diapir, or diatreme, along pre-existing fractures, and can be emplaced deep in crust (geology), the crust, or erupted at the surface.
Typical REE enriched deposits types forming in rift settings are carbonatites, and A- and M-Type granitoids. Near subduction zones, partial melting of the subducting plate within the asthenosphere (80 to 200 km depth) produces a volatile-rich magma (high concentrations of and water), with high concentrations of alkaline elements, and high element mobility that the rare earths are strongly partitioned into. This melt may also rise along pre-existing fractures, and be emplaced in the crust above the subducting slab or erupted at the surface. REE-enriched deposits forming from these melts are typically S-Type granitoids.
Alkaline magmas enriched with rare-earth elements include carbonatites, peralkaline granites (pegmatites), and nepheline syenite. Carbonatites crystallize from -rich fluids, which can be produced by partial melting of hydrous-carbonated lherzolite to produce a CO-rich primary magma, by fractional crystallization (geology), fractional crystallization of an alkaline primary magma, or by separation of a -rich immiscible liquid from. These liquids are most commonly forming in association with very deep Precambrian cratons, like the ones found in Africa and the Canadian Shield.
Ferrocarbonatites are the most common type of carbonatite to be enriched in REE, and are often emplaced as late-stage, brecciated pipes at the core of igneous complexes. They consist of fine-grained calcite and hematite, sometimes with significant concentrations of ankerite and minor concentrations of siderite. Large carbonatite deposits enriched in rare-earth elements include Mount Weld in Australia, Thor Lake in Canada, Zandkopsdrift in South Africa, and Mountain Pass rare earth mine, Mountain Pass in the USA.
Pegmatite, Peralkaline granites (A-Type granitoids) have very high concentrations of alkaline elements and very low concentrations of phosphorus; they are deposited at moderate depths in extensional zones, often as igneous ring complexes, or as pipes, massive bodies, and lenses. These fluids have very low viscosities and high element mobility, which allows for the crystallization of large grains, despite a relatively short crystallization time upon emplacement; their large grain size is why these deposits are commonly referred to as pegmatites.
Economically viable pegmatites include Niobium-Yttrium-Fluorine (NYF) types enriched in Yttrium and other rare-earth minerals, with REE-rich deposits found at Strange Lake in Canada and Khaladean-Buregtey in Mongolia. Nepheline syenite (M-Type granitoids) deposits are 90% feldspar and feldspathoid minerals. They are deposited in small, circular massifs and contain high concentrations of rare-earth mineral, rare-earth-bearing accessory minerals. For the most part, these deposits are small but important examples include Illimaussaq-Kvanefeld in Greenland, and Lovozera in Russia.
Rare-earth elements can also be enriched in deposits by secondary alteration either by interactions with hydrothermal fluids or meteoric water or by erosion and transport of resistate REE-bearing minerals. Argillization of primary minerals enriches insoluble elements by leaching out silica and other soluble elements, recrystallizing feldspar into clay minerals such kaolinite, halloysite, and montmorillonite. In tropical regions where precipitation is high, weathering forms a thick argillized regolith, this process is called supergene enrichment and produces laterite deposits. Heavy rare-earth elements are incorporated into the residual clay by absorption. This kind of deposit is only mined for REE in Southern China, where the majority of global heavy rare-earth element production occurs. REE-laterites do form elsewhere, including over the carbonatite at Mount Weld in Australia. REE may also be extracted from placer deposits if the sedimentary parent lithology contains REE-bearing, heavy resistate minerals.
In 2011, Yasuhiro Kato, a geologist at the University of Tokyo who led a study of Pacific Ocean seabed mud, published results indicating the mud could hold rich concentrations of rare-earth minerals. The deposits, studied at 78 sites, came from "[h]ot plumes from hydrothermal vents pull[ing] these materials out of seawater and deposit[ing] them on the seafloor, bit by bit, over tens of millions of years. One square patch of metal-rich mud 2.3 kilometers wide might contain enough rare earths to meet most of the global demand for a year, Japanese geologists report in ''Nature Geoscience''." "I believe that rare[-]earth resources undersea are much more promising than on-land resources," said Kato. "[C]oncentrations of rare earths were comparable to those found in clays mined in China. Some deposits contained twice as much heavy rare earths such as dysprosium, a component of magnets in hybrid car motors."Powell, Devin"Rare earth elements plentiful in ocean sediments"
''ScienceNews'', 3 July 2011. Via Kurt Brouwer'
Fundmastery Blog
, ''MarketWatch'', 2011-07-05. Retrieved 2011-07-05. The global demand for rare-earth elements (REEs) is expected to increase more than fivefold by 2030.
Geochemistry
The REE geochemical classification is usually done on the basis of their atomic weight. One of the most common classifications divides REE into 3 groups: light rare earths (LREE - from 57La to 60Nd), intermediate (MREE - from 62Sm to 67Ho) and heavy (HREE - from 68Er to 71Lu). REE usually appear as trivalent ions, except for Ce and Eu which can take the form of Ce4+ and Eu2+ depending on the redox conditions of the system. Consequentially, REE are characterized by a substantial identity in their chemical reactivity, which results in a serial behaviour during geochemical processes rather than being characteristic of a single element of the series. Sc, Y, and Lu can be electronically distinguished from the other rare earths because they do not have ''f'' valence electrons, whereas the others do, but the chemical behaviour is almost the same. A distinguishing factor in the geochemical behaviour of the REE is linked to the so-called "lanthanide contraction" which represents a higher-than-expected decrease in the atomic/ionic radius of the elements along the series. This is determined by the variation of the shielding effect towards the nuclear charge due to the progressive filling of the 4''f'' orbital which acts against the electrons of the 6''s'' and 5''d'' orbitals. The lanthanide contraction has a direct effect on the geochemistry of the lanthanides, which show a different behaviour depending on the systems and processes in which they are involved. The effect of the lanthanide contraction can be observed in the REE behaviour both in a CHARAC-type geochemical system (CHArge-and-RAdius-Controlled) where elements with similar charge and radius should show coherent geochemical behaviour, and in non-CHARAC systems, such as aqueous solutions, where the electron structure is also an important parameter to consider as the lanthanide contraction affects the ionic potential. A direct consequence is that, during the formation of coordination bonds, the REE behaviour gradually changes along the series. Furthermore, the lanthanide contraction causes the ionic radius of Ho3+ (0.901 Å) to be almost identical to that of Y3+ (0.9 Å), justifying the inclusion of the latter among the REE.Applications
The application of rare-earth elements to geology is important to understanding the petrological processes of igneous rock, igneous, sedimentary rock, sedimentary and metamorphic rock, metamorphic rock formation. In geochemistry, rare-earth elements can be used to infer the petrological mechanisms that have affected a rock due to the subtle atomic radius, atomic size differences between the elements, which causes preferential fractional crystallization (geology), fractionation of some rare earths relative to others depending on the processes at work. The geochemical study of the REE is not carried out on absolute concentrations – as it is usually done with other chemical elements – but on normalized concentrations in order to observe their serial behaviour. In geochemistry, rare-earth elements are typically presented in normalized "spider" diagrams, in which concentration of rare-earth elements are normalized to a reference standard and are then expressed as the logarithm to the base 10 of the value. Commonly, the rare-earth elements are normalized to chondrite, chondritic meteorites, as these are believed to be the closest representation of fractional crystallization (geology), unfractionated Solar System material. However, other normalizing standards can be applied depending on the purpose of the study. Normalization to a standard reference value, especially of a material believed to be unfractionated, allows the observed abundances to be compared to the initial abundances of the element. Normalization also removes the pronounced 'zig-zag' pattern caused by the differences in abundance between even and oddatomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
s. Normalization is carried out by dividing the analytical concentrations of each element of the series by the concentration of the same element in a given standard, according to the equation:
:
where ''n'' indicates the normalized concentration, the analytical concentration of the element measured in the sample, and the concentration of the same element in the reference material.
It is possible to observe the serial trend of the REE by reporting their normalized concentrations against the atomic number. The trends that are observed in "spider" diagrams are typically referred to as "patterns", which may be diagnostic of petrological processes that have affected the material of interest.
According to the general shape of the patterns or thanks to the presence (or absence) of so-called "anomalies", information regarding the system under examination and the occurring geochemical processes can be obtained. The anomalies represent enrichment (positive anomalies) or depletion (negative anomalies) of specific elements along the series and are graphically recognizable as positive or negative "peaks" along the REE patterns. The anomalies can be numerically quantified as the ratio between the normalized concentration of the element showing the anomaly and the predictable one based on the average of the normalized concentrations of the two elements in the previous and next position in the series, according to the equation:
:
where is the normalized concentration of the element whose anomaly has to be calculated, and the normalized concentrations of the respectively previous and next elements along the series.
The rare-earth elements patterns observed in igneous rocks are primarily a function of the chemistry of the source where the rock came from, as well as the fractionation history the rock has undergone. Fractionation is in turn a function of the partition coefficients of each element. Partition coefficients are responsible for the fractionation of trace elements (including rare-earth elements) into the liquid phase (the melt/magma) into the solid phase (the mineral). If an element preferentially remains in the solid phase it is termed 'compatible', and if it preferentially partitions into the melt phase it is described as 'incompatible'. Each element has a different partition coefficient, and therefore fractionates into solid and liquid phases distinctly. These concepts are also applicable to metamorphic and sedimentary petrology.
In igneous rocks, particularly in felsic melts, the following observations apply: anomalies in europium are dominated by the crystallization of feldspars. Hornblende, controls the enrichment of MREE compared to LREE and HREE. Depletion of LREE relative to HREE may be due to the crystallization of olivine, pyroxene, orthopyroxene, and pyroxene, clinopyroxene. On the other hand, the depletion of HREE relative to LREE may be due to the presence of garnet, as garnet preferentially incorporates HREE into its crystal structure. The presence of zircon may also cause a similar effect.
In sedimentary rocks, rare-earth elements in clastic rock, clastic sediments are a representation of provenance. The rare-earth element concentrations are not typically affected by sea and river waters, as rare-earth elements are insoluble and thus have very low concentrations in these fluids. As a result, when sediment is transported, rare-earth element concentrations are unaffected by the fluid and instead the rock retains the rare-earth element concentration from its source.
Sea and river waters typically have low rare-earth element concentrations. However, aqueous geochemistry is still very important. In oceans, rare-earth elements reflect input from rivers, hydrothermal vents, and aeolian processes, aeolian sources; this is important in the investigation of ocean mixing and circulation.
Rare-earth elements are also useful for dating rocks, as some radioactive isotopes display long half-lives. Of particular interest are the La-Ce, samarium-147, Sm-Nd, and Lu-Hf systems.
Production
Wikinvest. Retrieved on 11 Aug 2010. although it had only 36.7% of reserves. In 2018, Australia was the world's second largest producer, and the only other major producer, with 15% of world production. All of the world's heavy rare earths (such as dysprosium) come from Chinese rare-earth sources such as the polymetallic Bayan Obo deposit. The Browns Range mine, located 160 km south east of Halls Creek in northern Western Australia, was under development in 2018 and is positioned to become the first significant dysprosium producer outside of China. REE is increasing in demand due to the fact that they are essential for new and innovative technology that is being created. These new products that need REEs to be produced are high-technology equipment such as smart phones, digital cameras, computer parts, semiconductors, etc. In addition, these elements are more prevalent in the following industries: renewable energy technology, military equipment, glass making, and metallurgy. Increased demand has strained supply, and there is growing concern that the world may soon face a shortage of the rare earths. In 2009, future worldwide demand for rare-earth elements was expected to exceed supply by 40,000 metric tons annually unless major new sources are developed."As hybrid cars gobble rare metals, shortage looms"
. Reuters. August 31, 2009. Retrieved Aug 31, 2009. In 2013, it was stated that the demand for REEs would increase due to the dependence of the EU on these elements, the fact that rare-earth elements cannot be substituted by other elements and that REEs have a low recycling rate. Due to the increased demand and low supply, future prices are expected to increase and there is a chance that countries other than China will open REE mines. In 2023, there were over a hundred ongoing mining projects, with many options outside of China. As a result of the increased demand and tightening restrictions on exports of the metals from China, some countries are stockpiling rare-earth resources. Searches for alternative sources in Australia, Brazil, Canada, South Africa, Tanzania, Greenland, and the United States are ongoing. Mines in these countries were closed when China undercut world prices in the 1990s, and it will take a few years to restart production as there are many barriers to entry.
China
These concerns have intensified due to the actions of China, the predominant supplier. Specifically, China has announced regulations on exports and a crackdown on smuggling. On September 1, 2009, China announced plans to reduce its export quota to 35,000 tons per year in 2010–2015 to conserve scarce resources and protect the environment. On October 19, 2010, ''China Daily'', citing an unnamed Ministry of Commerce official, reported that China will "further reduce quotas for rare-earth exports by 30 percent at most next year to protect the precious metals from over-exploitation." The government in Beijing further increased its control by forcing smaller, independent miners to merge into state-owned corporations or face closure. At the end of 2010, China announced that the first round of export quotas in 2011 for rare earths would be 14,446 tons, which was a 35% decrease from the previous first round of quotas in 2010. China announced further export quotas on 14 July 2011 for the second half of the year with total allocation at 30,184 tons with total production capped at 93,800 metric tons. In September 2011, China announced the halt in production of three of its eight major rare-earth mines, responsible for almost 40% of China's total rare-earth production. In March 2012, the US, EU, and Japan confronted China at WTO about these export and production restrictions. China responded with claims that the restrictions had environmental protection in mind. In August 2012, China announced a further 20% reduction in production. The United States, Japan, and the European Union filed a joint lawsuit with the World Trade Organization in 2012 against China, arguing that China should not be able to deny such important exports. In 2012, in response to the opening of new mines in other countries (Lynas in Australia and Molycorp in the United States), prices of rare earths dropped. The price of dysprosium oxide was US$994/kg in 2011, and dropped to US$265/kg by 2014. In August 2014, the WTO ruled that China had broken free-trade agreements, and the WTO said in the summary of key findings that "the overall effect of the foreign and domestic restrictions is to encourage domestic extraction and secure preferential use of those materials by Chinese manufacturers." China declared that it would implement the ruling on September 26, 2014, but would need some time to do so. By January 5, 2015, China had lifted all quotas from the export of rare earths, but export licenses will still be required. In 2019, China supplied between 85% and 95% of the global demand for the 17 rare-earth powders, half of them sourced from Myanmar. After the 2021 Myanmar coup d'état, 2021 military coup in that country, future supplies of critical ores were possibly constrained. Additionally, it was speculated that the PRC could again reduce rare-earth exports to counter-act economic sanctions imposed by the US and EU countries. Rare-earth metals serve as crucial materials for electric vehicle manufacturing and high-tech military applications. In 2025, during the China–United States trade war, China restricted exports of heavy rare earths to the United States. Between 2020 and 2023, 70% of all rare earth compounds and metals imported into the United States came from China.Myanmar
Kachin State in Myanmar is the world's largest source of rare earths. In 2021, China imported of rare earths from Myanmar in December 2021, exceeding 20,000 metric tons. Rare earths were discovered near Pang War in Chipwi Township along the China–Myanmar border in the late 2010s. As China has shut down domestic mines due to the detrimental environmental impact, it has largely outsourced rare-earth mining to Kachin State. Chinese companies and miners illegally set up operations in Kachin State without government permits, and instead circumvent the central government by working with a Border Guard Forces, Border Guard Force militia under the Tatmadaw, formerly known as the New Democratic Army – Kachin, which has profited from this extractive industry. , 2,700 mining collection pools scattered across 300 separate locations were found in Kachin State, encompassing the area of Singapore, an exponential increase from 2016. Land has also been seized from locals to conduct mining operations.South Africa
Significant sites under development include Steenkampskraal mine, Steenkampskraal in South Africa, the world's highest grade rare earths and thorium mine, closed in 1963, but has been gearing to go back into production. Over 80% of the infrastructure is already complete.Tanzania
Adding to potential mine sites, Australian Securities Exchange, ASX listed Peak Resources announced in February 2012, that their Tanzanian-based Ngualla project contained not only the 6th largest deposit by tonnage outside of China but also the highest grade of rare-earth elements of the 6.Australia
Other mines include the Nolans Project in Central Australia, the Bokan Mountain project in Alaska, the remote Hoidas Lake project in northern Canada, and the Mount Weld project in Australia. The Hoidas Lake project has the potential to supply about 10% of the $1 billion of REE consumption that occurs in North America every year.Canada
Under consideration for mining are sites such as Thor Lake in the Northwest Territories.Vietnam
Vietnam signed an agreement in October 2010 to supply Japan with rare earths from its Tây Bắc, northwestern Lai Châu Province. The deal was never realized due to disagreements.USA
The largest rare-earth deposit in the U.S. is at Mountain Pass rare earth mine, Mountain Pass, California, sixty miles south of Las Vegas. Originally opened by Molycorp, the deposit has been mined, off and on, since 1951. A second large deposit of REEs at Elk Creek in southeast Nebraska is under consideration by NioCorp Development Ltd who hopes to open a niobium, scandium, and titanium mine there. That mine may be able to produce as much as 7,200 metric tons of ferro niobium and 95 metric tons of scandium trioxide annually. As of 2022, financing is still in the works. In 2024 American Rare Earths Inc. disclosed that its reserves near Wheatland Wyoming totaled 2.34 billion metric tons, possibly the world's largest and larger than a separate 1.2 million metric ton deposit in northeastern Wyoming.UK
In the UK, Pensana has begun construction of their US$195 million rare-earth processing plant which secured funding from the UK government's Automotive Transformation Fund. The plant will process ore from the Longonjo#Economy, Longonjo mine in Angola and other sources as they become available. The company are targeting production in late 2023, before ramping up to full capacity in 2024. Pensana aim to produce 12,500 metric tons of separated rare earths, including 4,500 metric tons of magnet metal rare earths.Greenland
In 2010, a large deposit of rare-earth minerals was discovered in Kvanefjeld in southern Greenland. Pre-feasibility drilling at this site has confirmed significant quantities of black lujavrite, which contains about 1% rare-earth oxides (REO). The European Union has urged Greenland to restrict Chinese development of rare-earth projects there, but as of early 2013, the government of Greenland has said that it has no plans to impose such restrictions. Many Danish politicians have expressed concerns that other nations, including China, could gain influence in thinly populated Greenland, given the number of foreign workers and investment that could come from Chinese companies in the near future because of the law passed December 2012.Spain
In central Spain, Province of Ciudad Real, Ciudad Real Province, the proposed rare-earth mining project 'Matamulas' may provide, according to its developers, up to 2,100 Tn/year (33% of the annual UE demand). However, this project has been suspended by regional authorities due to social and environmental concerns.North Korea
North Korea has been reported to have exported rare-earth ore to China, about US$1.88 million worth during May and June 2014.Japan
In May 2012, researchers from two universities in Japan announced that they had discovered rare earths in Ehime Prefecture, Japan.Sweden
In January 2023, Swedish state-owned mining company LKAB announced that it had discovered a deposit of over 1 million metric tons of rare earths in the country's Kiruna area, which would make it the largest such deposit in Europe. China processes about 90% of the world's REEs. As a result, the European Union imports practically all of its rare earth elements from China. The European Union Parliament considers this to a strategic risk.Norway
In June 2024, Rare Earths Norway found a rare-earth oxide deposit of 8.8 million metric tons in Telemark, Norway, making it Europe's largest known rare-earth element deposit. The mining firm predicted that it would finish developing the first stage of mining in 2030.Ukraine
Ukraine holds significant rare earth deposits, which have been at the center of the Russian invasion of Ukraine, Russian invasion of the country and peace negotiations.Malaysia
In early 2011, Australian mining company Lynas was reported to be "hurrying to finish" a US$230 million rare-earth refinery on the eastern coast of Peninsular Malaysia's industrial port of Kuantan. The plant would refine ore — lanthanides concentrate from the Mount Weld mine in Australia. The ore would be trucked to Fremantle and transported by container ship to Kuantan. Within two years, Lynas was said to expect the refinery to be able to meet nearly a third of the world's demand for rare-earth materials, not counting China. The Kuantan development brought renewed attention to the Malaysian town of Bukit Merah, Perak#District of Kinta, Central Perak, Bukit Merah in Perak, where a rare-earth mine operated by a Mitsubishi Chemical Holdings, Mitsubishi Chemical subsidiary, Asian Rare Earth, closed in 1994 and left #Environmental considerations, continuing environmental and health concerns. In mid-2011, after protests, Malaysian government restrictions on the Lynas plant were announced. At that time, citing subscription-only ''Dow Jones Newswire'' reports, a ''Barron's (newspaper), Barrons'' report said the Lynas investment was $730 million, and the projected share of the global market it would fill put at "about a sixth." An independent review initiated by the Malaysian Government, and conducted by the International Atomic Energy Agency (IAEA) in 2011 to address concerns of radioactive hazards, found no non-compliance with international radiation safety standards. However, the Malaysian authorities confirmed that as of October 2011, Lynas was not given any permit to import any rare-earth ore into Malaysia. In February 2012, the Malaysian AELB (Atomic Energy Licensing Board) recommended that Lynas be issued a temporary operating license subject to meeting a number of conditions. In September 2014, Lynas was issued a 2-year full operating stage license by the AELB. In November 2024, Minister of Economy (Malaysia), economy minister Rafizi Ramli said he hoped Malaysia is able to produce rare-earth elements within three years, through discussions with China to provide technology. In the past, plans to mine rare-earth elements at Kedah caused concerns of destroying forest reserves and harming water catchment areas.Other sources
Mine tailings
Significant quantities of rare-earth oxides are found in tailings accumulated from 50 years of uranium ore, shale, andloparite
Loparite-(Ce) is a granular, brittle oxide mineral of the perovskite class. It is black to dark grey and may appear grey to white in reflected light on polished thin section with reddish brown internal reflections. It has the chemical formula of . ...
mining at Sillamäe, Estonia. Due to the rising prices of rare earths, extraction of these oxides has become economically viable. The country currently exports around 3,000 metric tons per year, representing around 2% of world production. Similar resources are suspected in the western United States, where gold rush-era mines are believed to have discarded large amounts of rare earths, because they had no value at the time.
Ocean mining
In January 2013 a Japanese deep-sea research vessel obtained seven deep-sea mud core samples from the Pacific Ocean seafloor at 5,600 to 5,800 meters depth, approximately south of the island of Minami-Tori-Shima. The research team found a mud layer 2 to 4 meters beneath the seabed with concentrations of up to 0.66% rare-earth oxides. A potential deposit might compare in grade with the ion-absorption-type deposits in southern China that provide the bulk of Chinese REO mine production, which grade in the range of 0.05% to 0.5% REO.Waste and recycling
Another recently developed source of rare earths is electronic waste and other wastes that have significant rare-earth components. Advances in recycling, recycling technology have made the extraction of rare earths from these materials less expensive. Recycling plants operate in Japan, where an estimated 300,000 tons of rare earths are found in unused electronics. In France, the Rhodia (company), Rhodia group is setting up two factories, in La Rochelle and Saint-Fons, that will produce 200 tons of rare earths a year from used fluorescent lamps, magnets, and batteries. Coal and coal by-products, such as Coal combustion products, ash and sludge, are a potential source of critical elements including rare-earth elements (REE) with estimated amounts in the range of 50 million metric tons.Methods
A 2022 study mixed fly ash with carbon black and then sent a 1-second current pulse through the mixture, heating it to . The fly ash contains microscopic bits of glass that encapsulate the metals. The heat shatters the glass, exposing the rare earths. Flash heating also convertsphosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s into oxides, which are more soluble and extractable. Using hydrochloric acid at concentrations less than 1% of conventional methods, the process extracted twice as much material.
Properties
According to chemistry professor Andrea Sella in 2016, rare-earth elements differ from other elements, in that when looked at analytically, they are virtually inseparable, having almost the same chemical properties. However, in terms of their electronic and magnetic properties, each one occupies a unique technological niche that nothing else can.Professor of Chemistry at University College London, Andrea Sella, , Interview on TRT World / Oct 2016, minutes 4:40 - ff. For example, "the rare-earth elements praseodymium (Pr) and neodymium (Nd) can both be embedded inside glass and they completely cut out the glare from the flame when one is doing glass-blowing."Uses
The uses, applications, and demand for rare-earth elements have expanded over the years. Globally, most REEs are used for catalysis, catalysts and magnets. In the US, more than half of REEs are used for catalysts; ceramics, glass, and polishing are also main uses. Other important uses of rare-earth elements are applicable to the production of high-performance magnets, alloys, glasses, and electronics. Ce and La are important as catalysts, and are used for petroleum refining and as Diesel exhaust fluid, diesel additives. Nd is important in magnet production in traditional and low-carbon technologies. Rare-earth elements in this category are used in the electric motors of hybrid vehicle, hybrid and electric vehicles, generators in some wind turbines, hard disc drives, portable electronics, microphones, and speakers. Ce, La, and Nd are important in alloy making, and in the production of fuel cells and nickel–metal hydride battery, nickel-metal hydride batteries. Ce, Ga, and Nd are important in electronics and are used in the production of LCD and plasma screens, fiber optics, and lasers, and in medical imaging. Additional uses for rare-earth elements are as tracers in medical applications, fertilizers, and in water treatment. REEs have been used in agriculture to increase plant growth, productivity, and stress resistance seemingly without negative effects for human and animal consumption. REEs are used in agriculture through REE-enriched fertilizers which is a widely used practice in China. REEs are feed additives for livestock which has resulted in increased production such as larger animals and a higher production of eggs and dairy products. This practice has resulted in REE bioaccumulation within livestock and has impacted vegetation and algae growth in these agricultural areas. While no ill effects have been observed at current low concentrations, the effects over the long term and with accumulation over time are unknown, prompting some calls for more research into their possible effects. REEs also have applications in defense. The strength of neodynium magnets can be used in missile guidance systems. For high-end camera lenses used for intelligence, lanthanum enhances the clarity of the glass.Environmental considerations
REEs are naturally found in very low concentrations in the environment. Mines are often in countries where environmental and social standards are very low, leading to human rights violations, deforestation, and contamination of land and water. Generally, it is estimated that extracting 1 metric ton of rare earth element creates around 2,000 metric tons of waste, partly toxic, including 1 ton of radioactive waste. The largest mining site of REEs, Bayan Obo Mining District, Bayan Obo in China produced more than 70,000 tons of radioactive waste, that contaminated ground water. Near mining and industrial sites, the concentrations of REEs can rise to many times the normal background levels. Once in the environment, REEs can leach into the soil where their transport is determined by numerous factors such as erosion, weathering, pH, precipitation, groundwater, etc. Acting much like metals, they can speciate depending on the soil condition being either motile or adsorbed to soil particles. Depending on their bio-availability, REEs can be absorbed into plants and later consumed by humans and animals. The mining of REEs, use of REE-enriched fertilizers, and the production of phosphorus fertilizers all contribute to REE contamination. Strong acids are used during the extraction process of REEs, which can then leach out into the environment and be transported through water bodies and result in the acidification of aquatic environments. Another additive of REE mining that contributes to REE environmental contamination is cerium(IV) oxide, cerium oxide (), which is produced during the combustion of diesel and released as exhaust, contributing heavily to soil and water contamination. Mining, refining, and recycling of rare earths have serious environmental consequences if not properly managed. Low-level radioactive tailings resulting from the occurrence of
Mining, refining, and recycling of rare earths have serious environmental consequences if not properly managed. Low-level radioactive tailings resulting from the occurrence of thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
and uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
in rare-earth ores present a potential hazard and improper handling of these substances can result in extensive environmental damage. In May 2010, China announced a major, five-month crackdown on illegal mining in order to protect the environment and its resources. This campaign is expected to be concentrated in the South, where mines – commonly small, rural, and illegal operations – are particularly prone to releasing toxic waste into the general water supply.
The major operation in Baotou, in Inner Mongolia, where much of the world's rare-earth supply is refined, has caused major environmental damage. China's Ministry of Industry and Information Technology estimated that cleanup costs in Jiangxi province at $5.5 billion.
It is possible to filter out and recover any rare-earth elements that flow out with the wastewater from mining facilities. Such filtering and recovery equipment may not always be present on the outlets carrying the wastewater.
Recycling and reusing REEs
REEs are amongst the most critical elements to modern technologies and society. Despite this, typically only around 1% of REEs are recycled from end-products. Recycling and reusing REEs is not easy: these elements are mostly present in tiny amounts in small electronic parts and they are difficult to separate chemically. For example, recovery of neodymium requires manual disassembly of hard disk drives because shredding the drives only recovers 10% of the REE. REE recycling and reuse have been increasingly focused on in recent years. The main concerns include environmental pollution during REE recycling and increasing recycling efficiency. Literature published in 2004 suggests that, along with previously established pollution mitigation, a more circular supply chain would help mitigate some of the pollution at the extraction point. This means recycling and reusing REEs that are already in use or reaching the end of their life cycle. A study published in 2014 suggests a method to recycle REEs from waste nickel-metal hydride batteries, demonstrating a recovery rate of 95.16%. Rare-earth elements could also be recovered from industrial wastes with practical potential to reduce environmental and health impacts from mining, waste generation, and imports if known and experimental processes are scaled up. A 2019 study suggests that "fulfillment of the circular economy approach could reduce up to 200 times the impact Climate change mitigation, in the climate change category and up to 70 times the cost due to the REE mining." In 2020, in most of the reported studies reviewed by a scientific review, "secondary waste is subjected to chemical and or bioleaching followed by solvent extraction processes for clean separation of REEs." Currently, people take two essential resources into consideration for the secure supply of REEs: one is to extract REEs from primary resources like mines harboring REE-bearing ores, regolith-hosted clay deposits, ocean bed sediments, coal fly ash, etc. A work developed a green system for recovery of REEs from coal fly ash by using citrate and oxalate who are strong organic ligand and capable of complexing or precipItating with REE. The other one is from secondary resources such as electronic, industrial waste and municipal waste. E-waste contains a significant concentration of REEs, and thus is the primary option for REE recycling now. According to a 2019 study, approximately 50 million metric tons of electronic waste are dumped in landfills worldwide each year. Despite the fact that e-waste contains a significant amount of rare-earth elements (REE), only 12.5% of e-waste is currently being recycled for all metals.Impact of REE contamination
On vegetation
The mining of REEs has caused the soil contamination, contamination of soil and water around production areas, which has impacted vegetation in these areas by decreasing chlorophyll production, which affects photosynthesis and inhibits the growth of the plants. However, the impact of REE contamination on vegetation is dependent on the plants present in the contaminated environment: not all plants retain and absorb REEs. Also, the ability of the vegetation to intake the REE is dependent on the type of REE present in the soil, hence there are a multitude of factors that influence this process. Agricultural plants are the main type of vegetation affected by REE contamination in the environment, the two plants with a higher chance of absorbing and storing REEs being apples and beets. There is a possibility that REEs can leach out into aquatic environments and be absorbed by aquatic vegetation, which can then bio-accumulate and potentially enter the human food chain if livestock or humans choose to eat the vegetation. An example of this situation was the case of the Eichhornia crassipes, water hyacinth (''Eichhornia crassipes)'' in China, where the water was contaminated due to a REE-enriched fertilizer being used in a nearby agricultural area. The aquatic environment became contaminated withcerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
and resulted in the water hyacinth becoming three times more concentrated in cerium than its surrounding water.
On human health
The chemical properties of the REEs are so similar that they are expected to show similar toxicity in humans. Mortality studies show REEs are not highly toxic. Long term (18 months) inhalation of dust containing high levels (60%) of REEs has been shown to cause pneumoconiosis but the mechanism is unknown. While REEs are not major pollutants, the increase application of REEs in new technologies has increased the need to understand their safe levels of exposure for humans. One side effect of mining REEs can be exposure to harmful radioactive Thorium as has been demonstrated at large mine in Batou (Mongolia). The rare-earth mining and smelting process can release airborne fluoride which will associate with total suspended particles (TSP) to form aerosols that can enter human respiratory systems. Research from Baotou, China shows that the fluoride concentration in the air near REE mines is higher than the limit value from WHO, but the health effects of this exposure are unknown. Analysis of people living near mines in China had many times the levels of REEs in their blood, urine, bone, and hair compared to controls far from mining sites, suggesting possible bioaccumulation of REEs. This higher level was related to the high levels of REEs present in the vegetables they cultivated, the soil, and the water from the wells, indicating that the high levels were caused by the nearby mine. However the levels found were not high enough to cause health effects. Analysis of REEs in street dust in China suggest "no augmented health hazard". Similarly, analysis of cereal crops in mining areas in China found levels too low for health risks.On animal health
Experiments exposing rats to various cerium compounds have found accumulation primarily in the lungs and liver. This resulted in various negative health outcomes associated with those organs. REEs have been added to feed in livestock to increase their body mass and increase milk production. They are most commonly used to increase the body mass of pigs, and it was discovered that REEs increase the digestibility and nutrient use of pigs' digestive systems. Studies point to a dose-response when considering toxicity versus positive effects. While small doses from the environment or with proper administration seem to have no ill effects, larger doses have been shown to have negative effects specifically in the organs where they accumulate. The process of mining REEs in China has resulted in soil and water contamination in certain areas, which when transported into aquatic bodies could potentially bio-accumulate within aquatic biota. In some cases, animals that live in REE-contaminated areas have been diagnosed with organ or system problems. REEs have been used in freshwater fish farming because it protects the fish from possible diseases. One main reason why they have been avidly used in animal livestock feeding is that they have had better results than inorganic livestock feed enhancers.Remediation after pollution
After the 1982 Bukit Merah radioactive pollution, the mine in Malaysia has been the focus of a US$100 million cleanup that is proceeding in 2011. After having accomplished the hilltop entombment of 11,000 truckloads of radioactively contaminated material, the project is expected to entail in summer, 2011, the removal of "more than 80,000 steel barrels of radioactive waste to the hilltop repository." In May 2011, after the Fukushima nuclear disaster, widespread protests took place in Kuantan over the #Production, Lynas refinery and radioactive waste from it. The ore to be processed has very low levels of thorium, and Lynas founder and chief executive Nicholas Curtis said "There is absolutely no risk to public health." T. Jayabalan, a doctor who says he has been monitoring and treating patients affected by the Mitsubishi plant, "is wary of Lynas's assurances. The argument that low levels of thorium in the ore make it safer doesn't make sense, he says, because radiation exposure is cumulative."Lee, Yoolim"Malaysia Rare Earths in Largest Would-Be Refinery Incite Protest"
, ''Bloomberg L.P., Bloomberg Markets Magazine'', May 31, 2011 5:00 PM ET. Construction of the facility has been halted until an independent United Nations IAEA panel investigation is completed, which is expected by the end of June 2011. #Production, New restrictions were announced by the Malaysian government in late June. An IAEA panel investigation was completed and no construction has been halted. Lynas is on budget and on schedule to start producing in 2011. The IAEA concluded in a report issued in June 2011 that it did not find any instance of "any non-compliance with international radiation safety standards" in the project. If the proper safety standards are followed, REE mining is relatively low impact. Molycorp (before going bankrupt) often exceeded environmental regulations to improve its public image. In Greenland, there is a significant dispute on whether to start a new rare-earth mine in Kvanefjeld due to environmental concerns.
Geopolitical considerations
In popular culture
The plot of Eric Ambler's now-classic 1967 international crime-thriller ''Dirty Story (novel), Dirty Story'', aka ''This Gun for Hire'', not to be confused with the 1942 movie ''This Gun for Hire'', features a struggle between two rival mining cartels to control a plot of land in a fictional African country, which contains rich minable rare-earth ore deposits.See also
* List of elements facing shortage * Material passport: lists used materials in products * Pensana Salt End * Precious metal * Rare-earth magnet * Rare-earth mineralReferences
External links
* * {{Authority control Rare earth elements, Metallic elements Sets of chemical elements