Protein translocation on:
[Wikipedia]
[Google]
[Amazon]
Protein targeting or protein sorting is the biological mechanism by which
 In 1970, Günter Blobel conducted experiments on protein translocation across
In 1970, Günter Blobel conducted experiments on protein translocation across
 Most secretory and membrane-bound proteins are co-translationally translocated. Proteins that reside in the
Most secretory and membrane-bound proteins are co-translationally translocated. Proteins that reside in the
 While some proteins in the mitochondria originate from
While some proteins in the mitochondria originate from
 Proteins targeted to the mitochondrial matrix first involves interactions between the matrix targeting sequence located at the N-terminus and the outer membrane import receptor complex TOM20/22. In addition to the docking of internal sequences and
Proteins targeted to the mitochondrial matrix first involves interactions between the matrix targeting sequence located at the N-terminus and the outer membrane import receptor complex TOM20/22. In addition to the docking of internal sequences and


Phobius
predicts signal peptides based on a supplied primary sequence.
SignalP
predicts signal peptide cleavage sites.
LOCtree
predicts the subcellular localization of proteins.
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle
In cell biology, an organelle is a specialized subunit, usually within a cell (biology), cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as Organ (anatomy), organs are to th ...
, different intracellular membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
s, the plasma membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
, or to the exterior of the cell via secretion
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mec ...
. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases.
History
membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
s. Blobel, then an assistant professor at Rockefeller University
The Rockefeller University is a Private university, private Medical research, biomedical Research university, research and graduate-only university in New York City, New York. It focuses primarily on the biological and medical sciences and pro ...
, built upon the work of his colleague George Palade. Palade had previously demonstrated that non-secreted proteins were translated by free ribosome
Ribosomes () are molecular machine, macromolecular machines, found within all cell (biology), cells, that perform Translation (biology), biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order s ...
s in the cytosol, while secreted proteins (and target proteins, in general) were translated by ribosomes bound to the endoplasmic reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for ...
(ER). Candidate explanations at the time postulated a processing difference between free and ER-bound ribosomes, but Blobel hypothesized that protein targeting relied on characteristics inherent to the proteins, rather than a difference in ribosomes. Supporting his hypothesis, Blobel discovered that many proteins have a short amino acid sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthe ...
at one end that functions like a postal code specifying an intracellular or extracellular destination. He described these short sequences (generally 13 to 36 amino acids residues) as signal peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16–30 amino acids long) present at the ...
s or signal sequences and was awarded the 1999 Nobel prize in Physiology for the same.
Signal peptides
Signal peptides serve as targeting signals, enabling cellular transport machinery to direct proteins to specific intracellular or extracellular locations. While noconsensus sequence
In molecular biology and bioinformatics, the consensus sequence (or canonical sequence) is the calculated sequence of most frequent residues, either nucleotide or amino acid, found at each position in a sequence alignment. It represents the result ...
has been identified for signal peptides, many nonetheless possess a characteristic tripartite structure:
# A positively charged, hydrophilic region near the N-terminal.
# A span of 10 to 15 hydrophobic amino acids near the middle of the signal peptide.
# A slightly polar region near the C-terminal, typically favoring amino acids with smaller side chains at positions approaching the cleavage site.
After a protein has reached its destination, the signal peptide is generally cleaved by a signal peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini.
Signal peptidases were initially observed in endoplasmic reticulum (ER)-deri ...
. Consequently, most mature proteins do not contain signal peptides. While most signal peptides are found at the N-terminal, in peroxisome
A peroxisome () is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen perox ...
s the targeting sequence is located on the C-terminal extension. Unlike signal peptides, signal patches are composed by amino acid residues that are discontinuous in the primary sequence but become functional when folding brings them together on the protein surface. Unlike most signal sequences, signal patches are not cleaved after sorting is complete. In addition to intrinsic signaling sequences, protein modifications like glycosylation can also induce targeting to specific intracellular or extracellular regions.
Protein translocation
Since thetranslation
Translation is the communication of the semantics, meaning of a #Source and target languages, source-language text by means of an Dynamic and formal equivalence, equivalent #Source and target languages, target-language text. The English la ...
of mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein.
mRNA is ...
into protein by a ribosome
Ribosomes () are molecular machine, macromolecular machines, found within all cell (biology), cells, that perform Translation (biology), biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order s ...
takes place within the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
, proteins destined for secretion or a specific organelle
In cell biology, an organelle is a specialized subunit, usually within a cell (biology), cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as Organ (anatomy), organs are to th ...
must be translocated. This process can occur during translation, known as co-translational translocation, or after translation is complete, known as post-translational translocation.
Co-translational translocation
 Most secretory and membrane-bound proteins are co-translationally translocated. Proteins that reside in the
Most secretory and membrane-bound proteins are co-translationally translocated. Proteins that reside in the endoplasmic reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for ...
(ER), golgi or endosome
Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of the endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membra ...
s also use the co-translational translocation pathway. This process begins while the protein is being synthesized on the ribosome, when a signal recognition particle
The signal recognition particle (SRP) is an abundant, cytosolic, universally conserved ribonucleoprotein (protein-RNA complex) that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane ...
(SRP) recognizes an N-terminal signal peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16–30 amino acids long) present at the ...
of the nascent protein. Binding of the SRP temporarily pauses synthesis while the ribosome-protein complex is transferred to an SRP receptor on the ER in eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of ...
, and the plasma membrane in prokaryotes
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ...
. There, the nascent protein is inserted into the translocon
The translocon (also known as a translocator or translocation channel) is a complex of proteins associated with the translocation of polypeptides across membranes. In eukaryotes the term translocon most commonly refers to the complex that transpor ...
, a membrane-bound protein conducting channel composed of the Sec61 translocation complex in eukaryotes, and the homologous SecYEG complex in prokaryotes. In secretory proteins and type I transmembrane proteins
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently un ...
, the signal sequence is immediately cleaved from the nascent polypeptide once it has been translocated into the membrane of the ER (eukaryotes) or plasma membrane (prokaryotes) by signal peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini.
Signal peptidases were initially observed in endoplasmic reticulum (ER)-deri ...
. The signal sequence of type II membrane proteins and some polytopic membrane proteins are not cleaved off and therefore are referred to as signal anchor sequences. Within the ER, the protein is first covered by a chaperone protein to protect it from the high concentration of other proteins in the ER, giving it time to fold correctly. Once folded, the protein is modified as needed (for example, by glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not ...
), then transported to the Golgi for further processing and goes to its target organelles or is retained in the ER by various ER retention mechanisms.
The amino acid chain of transmembrane proteins
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently un ...
, which often are transmembrane receptors, passes through a membrane one or several times. These proteins are inserted into the membrane by translocation, until the process is interrupted by a stop-transfer sequence, also called a membrane anchor or signal-anchor sequence. These complex membrane proteins are currently characterized using the same model of targeting that has been developed for secretory proteins. However, many complex multi-transmembrane proteins contain structural aspects that do not fit this model. Seven transmembrane G-protein coupled receptors (which represent about 5% of the genes in humans) mostly do not have an amino-terminal signal sequence. In contrast to secretory proteins, the first transmembrane domain acts as the first signal sequence, which targets them to the ER membrane. This also results in the translocation of the amino terminus of the protein into the ER membrane lumen. This translocation, which has been demonstrated with opsin
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become retinylidene proteins, but are usually still called opsins regardless. Most pro ...
with in vitro experiments, breaks the usual pattern of "co-translational" translocation which has always held for mammalian proteins targeted to the ER. A great deal of the mechanics of transmembrane topology and folding remains to be elucidated.
Post-translational translocation
Even though most secretory proteins are co-translationally translocated, some are translated in thecytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
and later transported to the ER/plasma membrane by a post-translational system. In prokaryotes this process requires certain cofactors such as SecA and SecB and is facilitated by Sec62 and Sec63, two membrane-bound proteins. The Sec63 complex, which is embedded in the ER membrane, causes hydrolysis of ATP, allowing chaperone proteins to bind to an exposed peptide chain and slide the polypeptide into the ER lumen. Once in the lumen the polypeptide chain can be folded properly. This process only occurs in unfolded proteins located in the cytosol.
In addition, proteins targeted to other cellular destinations, such as mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
, chloroplasts
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
, or peroxisomes
A peroxisome () is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen pe ...
, use specialized post-translational pathways. Proteins targeted for the nucleus are also translocated post-translationally through the addition of a nuclear localization sequence
A nuclear localization signal ''or'' sequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines ...
(NLS) that promotes passage through the nuclear envelope
The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material.
The nuclear envelope consists of two lipid bilayer membran ...
via nuclear pore
The nuclear pore complex (NPC), is a large protein complex giving rise to the nuclear pore. A great number of nuclear pores are studded throughout the nuclear envelope that surrounds the eukaryote cell nucleus. The pores enable the nuclear tran ...
s.
Sorting of proteins
Mitochondria

 While some proteins in the mitochondria originate from
While some proteins in the mitochondria originate from mitochondrial DNA
Mitochondrial DNA (mtDNA and mDNA) is the DNA located in the mitochondrion, mitochondria organelles in a eukaryotic cell that converts chemical energy from food into adenosine triphosphate (ATP). Mitochondrial DNA is a small portion of the D ...
within the organelle, most mitochondrial
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used ...
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s are synthesized as cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
precursors containing uptake peptide signals. Unfolded proteins bound by cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
chaperone hsp70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms and play crucial roles in the development of can ...
that are targeted to the mitochondria may be localized to four different areas depending on their sequences. They may be targeted to the mitochondrial matrix
In the mitochondrion, the matrix is the space within the inner membrane. It can also be referred as the mitochondrial fluid. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitoc ...
, the outer membrane, the intermembrane space
The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the Inner mitochondrial membrane, inner membrane and the Outer mitochondrial memb ...
, or the inner membrane. Defects in any one or more of these processes has been linked to health and disease.
Mitochondrial matrix
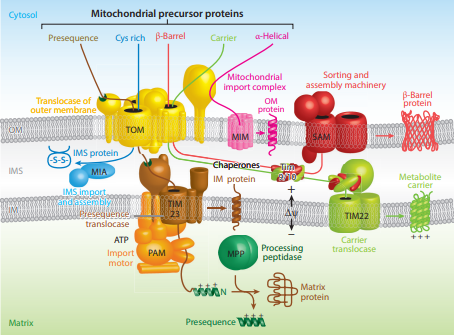
Proteins destined for the mitochondrial matrix have specific signal sequences at their beginning (N-terminus) that consist of a string of 20 to 50 amino acids. These sequences are designed to interact with receptors that guide the proteins to their correct location inside the mitochondria. The sequences have a unique structure with clusters of water-loving (hydrophilic) and water-avoiding (hydrophobic) amino acids, giving them a dual nature known as amphipathic. These amphipathic sequences typically form a spiral shape (alpha-helix) with the charged amino acids on one side and the hydrophobic ones on the opposite side. This structural feature is essential for the sequence to function correctly in directing proteins to the matrix. If mutations occur that mess with this dual nature, the protein often fails to reach its intended destination, although not all changes to the sequence have this effect. This indicates the importance of the amphipathic property for the protein to be correctly targeted to the mitochondrial matrix. Proteins targeted to the mitochondrial matrix first involves interactions between the matrix targeting sequence located at the N-terminus and the outer membrane import receptor complex TOM20/22. In addition to the docking of internal sequences and
Proteins targeted to the mitochondrial matrix first involves interactions between the matrix targeting sequence located at the N-terminus and the outer membrane import receptor complex TOM20/22. In addition to the docking of internal sequences and cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
chaperones to TOM70. Where TOM is an abbreviation for translocase of the outer membrane. Binding of the matrix targeting sequence to the import receptor triggers a handoff of the polypeptide to the general import core (GIP) known as TOM40. The general import core (TOM40) then feeds the polypeptide chain through the intermembrane space and into another translocase complex TIM17/23/44 located on the inner mitochondrial membrane. This is accompanied by the necessary release of the cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
chaperones that maintain an unfolded state prior to entering the mitochondria. As the polypeptide enters the matrix, the signal sequence is cleaved by a processing peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do ...
and the remaining sequences are bound by mitochondrial chaperones to await proper folding and activity. The push and pull of the polypeptide from the cytosol to the intermembrane space and then the matrix is achieved by an electrochemical gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts:
* The chemical gradient, or difference in Concentration, solute concentration across ...
that is established by the mitochondrion during oxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
. In which a mitochondrion active in metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
has generated a negative potential inside the matrix and a positive potential in the intermembrane space. It is this negative potential inside the matrix that directs the positively charged regions of the targeting sequence into its desired location.
Mitochondrial inner membrane
Targeting of mitochondrial proteins to the inner membrane may follow 3 different pathways depending upon their overall sequences, however, entry from the outer membrane remains the same using the import receptor complex TOM20/22 and TOM40 general import core. The first pathway for proteins targeted to the inner membrane follows the same steps as those designated to the matrix where it contains a matrix targeting sequence that channels the polypeptide to the inner membrane complex containing the previously mentioned translocase complex TIM17/23/44. However, the difference is that the peptides that are designated to the inner membrane and not the matrix contain an upstream sequence called the stop-transfer-anchor sequence. This stop-transfer-anchor sequence is a hydrophobic region that embeds itself into thephospholipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a l ...
of the inner membrane and prevents translocation further into the mitochondrion. The second pathway for proteins targeted to the inner membrane follows the matrix localization pathway in its entirety. However, instead of a stop-transfer-anchor sequence, it contains another sequence that interacts with an inner membrane protein called Oxa-1 once inside the matrix that will embed it into the inner membrane. The third pathway for mitochondrial proteins targeted to the inner membrane follow the same entry as the others into the outer membrane, however, this pathway utilizes the translocase complex TIM22/54 assisted by complex TIM9/10 in the intermembrane space to anchor the incoming peptide into the membrane. The peptides for this last pathway do not contain a matrix targeting sequence, but instead contain several internal targeting sequences.
Mitochondrial intermembrane space
If instead the precursor protein is designated to the intermembrane space of the mitochondrion, there are two pathways this may occur depending on the sequences being recognized. The first pathway to the intermembrane space follows the same steps for an inner membrane targeted protein. However, once bound to the inner membrane theC-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein
Proteins are large biomolecules and macromolecules that comp ...
of the anchored protein is cleaved via a peptidase that liberates the preprotein into the intermembrane space so it can fold into its active state. One of the greatest examples for a protein that follows this pathway is cytochrome b2, that upon being cleaved will interact with a heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
cofactor and become active. The second intermembrane space pathway does not utilize any inner membrane complexes and therefor does not contain a matrix targeting signal. Instead, it enters through the general import core TOM40 and is further modified in the intermembrane space to achieve its active conformation. TIM9/10 is an example of a protein that follows this pathway in order to be in the location it needs to be to assist in inner membrane targeting.
Mitochondrial outer membrane
Outer membrane targeting simply involves the interaction of precursor proteins with the outer membrane translocase complexes that embeds it into the membrane via internal-targeting sequences that are to form hydrophobicalpha helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
or beta barrels that span the phospholipid bilayer. This may occur by two different routes depending on the preprotein internal sequences. If the preprotein contains internal hydrophobic regions capable of forming alpha helices, then the preprotein will utilize the mitochondrial import complex (MIM) and be transferred laterally to the membrane. For preproteins containing hydrophobic internal sequences that correlate to beta-barrel forming proteins, they will be imported from the aforementioned outer membrane complex TOM20/22 to the intermembrane space. In which they will interact with TIM9/10 intermembrane-space protein complex that transfers them to sorting and assembly machinery (SAM) that is present in the outer membrane that laterally displaces the targeted protein as a beta-barrel.
Chloroplasts
Chloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
s are similar to mitochondria in that they contain their own DNA for production of some of their components. However, the majority of their proteins are obtained via post-translational translocation and arise from nuclear genes. Proteins may be targeted to several sites of the chloroplast depending on their sequences such as the outer envelope, inner envelope, stroma, thylakoid lumen, or the thylakoid membrane. Proteins are targeted to Thylakoids by mechanisms related to Bacterial Protein Translocation. Proteins targeted to the envelope of chloroplasts usually lack cleavable sorting sequence and are laterally displaced via membrane sorting complexes. General import for the majority of preproteins requires translocation from the cytosol through the Toc and Tic complexes located within the chloroplast envelope. Where Toc is an abbreviation for the translocase of the outer chloroplast envelope and Tic is the translocase of the inner chloroplast envelope. There is a minimum of three proteins that make up the function of the Toc complex. Two of which, referred to as Toc159 and Toc34, are responsible for the docking of stromal import sequences and both contain GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a ...
activity. The third known as Toc 75, is the actual translocation channel that feeds the recognized preprotein by Toc159/34 into the chloroplast.
Stroma
Targeting to the stroma requires the preprotein to have a stromal import sequence that is recognized by the Tic complex of the inner envelope upon being translocated from the outer envelope by the Toc complex. The Tic complex is composed of at least five different Tic proteins that are required to form the translocation channel across the inner envelope. Upon being delivered to the stroma, the stromal import sequence is cleaved off via a signal peptidase. This delivery process to the stroma is currently known to be driven by ATP hydrolysis via stromal HSP chaperones, instead of the transmembraneelectrochemical gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts:
* The chemical gradient, or difference in Concentration, solute concentration across ...
that is established in mitochondria to drive protein import. Further intra-chloroplast sorting depends on additional target sequences such as those designated to the thylakoid membrane
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thyl ...
or the thylakoid lumen
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thyla ...
.
Thylakoid lumen
If a protein is to be targeted to the thylakoid lumen, this may occur via four differently known routes that closely resemble bacterial protein transport mechanisms. The route that is taken depends upon the protein delivered to the stroma being in either an unfolded or metal-bound folded state. Both of which will still contain a thylakoid targeting sequence that is also cleaved upon entry to the lumen. While protein import into the stroma is ATP-driven, the pathway for metal-bound proteins in a folded state to the thylakoid lumen has been shown to be driven by a pH gradient.
Thylakoid membrane
Proteins bound for the membrane of the thylakoid will follow up to four known routes that are illustrated in the corresponding figure shown. They may follow a co-translational insertion route that utilizes stromal ribosomes and the SecY/E transmembrane complex, the SRP-dependent pathway, the spontaneous insertion pathway, or the GET pathway. The last of the three are post-translational pathways originating from nuclear genes and therefor constitute the majority of proteins targeted to the thylakoid membrane. According to recent review articles in the journal of biochemistry and molecular biology, the exact mechanisms are not yet fully understood.Both chloroplasts and mitochondria
Many proteins are needed in both mitochondria and chloroplasts. In general the dual-targeting peptide is of intermediate character to the two specific ones. The targeting peptides of theseprotein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s have a high content of basic and hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
, a low content of negatively charged amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
. They have a lower content of alanine and a higher content of leucine and phenylalanine. The dual targeted proteins have a more hydrophobic targeting peptide than both mitochondrial and chloroplastic ones. However, it is tedious to predict if a peptide is dual-targeted or not based on its physio-chemical characteristics.
Nucleus
The nucleus of a cell is surrounded by a nuclear envelope consisting of two layers, with the inner layer providing structural support and anchorage for chromosomes and the nuclear lamina. The outer layer is similar to the endoplasmic reticulum (ER) membrane. This envelope contains nuclear pores, which are complex structures made from around 30 different proteins. These pores act as selective gates that control the flow of molecules into and out of the nucleus. While small molecules can pass through these pores without issue, larger molecules, like RNA and proteins destined for the nucleus, must have specific signals to be allowed through. These signals are known as nuclear localization signals, usually comprising short sequences rich in positively charged amino acids like lysine or arginine. Proteins called nuclear import receptors recognize these signals and guide the large molecules through the nuclear pores by interacting with the disordered, mesh-like proteins that fill the pore. The process is dynamic, with the receptor moving the molecule through the meshwork until it reaches the nucleus. Once inside, a GTPase enzyme called Ran, which can exist in two different forms (one bound to GTP and the other to GDP), facilitates the release of the cargo inside the nucleus and recycles the receptor back to the cytosol. The energy for this transport comes from the hydrolysis of GTP by Ran. Similarly, nuclear export receptors help move proteins and RNA out of the nucleus using a different signal and also harnessing Ran's energy conversion. Overall, the nuclear pore complex works efficiently to transport macromolecules at high speed, allowing proteins to move in their folded state and ribosomal components as complete particles, which is distinct from how proteins are transported into most other organelles.Endoplasmic reticulum
The endoplasmic reticulum (ER) plays a key role in protein synthesis and distribution in eukaryotic cells. It's a vast network of membranes where proteins are processed and sorted to various destinations, including the ER itself, the cell surface, and other organelles like the Golgi apparatus, endosomes, and lysosomes. Unlike other organelle-targeted proteins, those headed for the ER start to be transferred across its membrane while they're still being made.Protein synthesis and sorting
There are two types of proteins that move to the ER: water-soluble proteins, which completely cross into the ER lumen, and transmembrane proteins, which partly cross and embed themselves within the ER membrane. These proteins find their way to the ER with the help of an ER signal sequence, a short stretch of hydrophobic amino acids. Proteins entering the ER are synthesized by ribosomes. There are two sets of ribosomes in the cell: those bound to the ER (making it look 'rough') and those floating freely in the cytosol. Both sets are identical but differ in the proteins they synthesize at a given moment. Ribosomes that are making proteins with an ER signal sequence attach to the ER membrane and start the translocation process. This process is energy-efficient because the growing protein chain itself pushes through the ER membrane as it elongates. As the mRNA is translated into a protein, multiple ribosomes may attach to it, creating a structure called a polyribosome. If the mRNA is coding for a protein with an ER signal sequence, the polyribosome attaches to the ER membrane, and the protein begins to enter the ER while it is still being synthesized.= Guided entry of soluble proteins
= In the process of protein synthesis within eukaryotic cells, soluble proteins that are destined for the endoplasmic reticulum (ER) or for secretion out of the cell are guided to the ER by a two-part system. Firstly, a signal-recognition particle (SRP) in the cytosol attaches to the emerging protein's ER signal sequence and the ribosome itself. Secondly, an SRP receptor located in the ER membrane recognizes and binds to the SRP. This interaction temporarily slows down protein synthesis until the SRP and ribs complex binds to the SRP receptor on the ER. Once this binding occurs, the SRP is released, and the ribosome is transferred to a protein translocator in the ER membrane, allowing protein synthesis to continue. The polypeptide chain of the protein is then threaded through a channel in the translocator into the ER lumen. The signal sequence of the protein, typically at the beginning (N-terminus) of the polypeptide chain, plays a dual role. It not only targets the ribosome to the ER but also triggers the opening of the translocator. As the protein is fed through the translocator, the signal sequence stays attached, allowing the rest of the protein to move through as a loop. A signal peptidase inside the ER then cuts off the signal sequence, which is subsequently discarded into the lipid bilayer of the ER membrane and broken down. Finally, once the last part of the protein (the C-terminus) passes through the translocator, the entire soluble protein is released into the ER lumen, where it can then fold and undergo further modifications or be transported to its final destination.Mechanisms of transmembrane protein integration Transmembrane proteins, which are partly integrated into the ER membrane rather than released into the ER lumen, have a complex assembly process. The initial stages are similar to soluble proteins: a signal sequence starts the insertion into the ER membrane. However, this process is interrupted by a stop-transfer sequence—a string of hydrophobic amino acids—which causes the translocator to halt and release the protein laterally into the membrane. This results in a single-pass transmembrane protein with one end inside the ER lumen and the other in the cytosol, and this orientation is permanent. Some transmembrane proteins use an internal signal (start-transfer sequence) instead of one at the N-terminus, and unlike the initial signal sequence, this start-transfer sequence isn't removed. It begins the transfer process, which continues until a stop-transfer sequence is encountered, at which point both sequences become anchored in the membrane as alpha-helical segments. In more complex proteins that span the membrane multiple times, additional pairs of start- and stop-transfer sequences are used to weave the protein into the membrane in a fashion akin to a sewing machine. Each pair allows a new segment to cross the membrane and adds to the protein's structure, ensuring it is properly embedded with the correct arrangement of segments inside and outside the ER membrane.
Peroxisomes

Peroxisome
A peroxisome () is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen perox ...
s contain a single phospholipid bilayer that surrounds the peroxisomal matrix containing a wide variety of proteins and enzymes that participate in anabolism and catabolism. Peroxisomes are specialized cell organelles that carry out specific oxidative reactions using molecular oxygen. Their primary function is to remove hydrogen atoms from organic molecules, a process that results in the production of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
(). Within peroxisomes, an enzyme called catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting ...
plays a critical role. It uses the hydrogen peroxide generated in the earlier reaction to oxidize various other substances, including phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
, formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, and alcohol. This is known as the "peroxidative" reaction.
Peroxisomes are particularly important in liver and kidney cells for detoxifying harmful substances that enter the bloodstream. For example, they are responsible for oxidizing about 25% of the ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
we consume into acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
. Additionally, catalase within peroxisomes can break down excess hydrogen peroxide into water and oxygen and thus preventing potential damage from the build-up of . Since it contains no internal DNA like that of the mitochondria or chloroplast all peroxisomal proteins are encoded by nuclear genes. To date there are two types of known Peroxisome Targeting Signals (PTS):
# Peroxisome targeting signal 1 (PTS1): a C-terminal tripeptide with a consensus sequence (S/A/C)-(K/R/H)-(L/A). The most common PTS1 is serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
-lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
-leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
(SKL). The initial research that led to the discovery of this consensus observed that when firefly luciferase was expressed in cultured insect cells it was targeted to the peroxisome. By testing a variety of mutations in the gene encoding the expressed luciferase
Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence, and is usually distinguished from a photoprotein. The name was first used by Raphaël Dubois who invented the words ''luciferin'' and ''luciferase'' ...
, the consensus sequence was then determined. It has also been found that by adding this C-terminal sequence of SKL to a cytosolic protein that it becomes targeted for transport to the peroxisome. The majority of peroxisomal matrix proteins possess this PTS1 type signal.
# Peroxisome targeting signal 2 (PTS2): a nonapeptide located near the N-terminus with a consensus sequence (R/K)-(L/V/I)-XXXXX-(H/Q)-(L/A/F) (where X can be any amino acid).
There are also proteins that possess neither of these signals. Their transport may be based on a so-called "piggy-back" mechanism: such proteins associate with PTS1-possessing matrix proteins and are translocated into the peroxisomal matrix together with them.
In the case of cytosolic proteins that are produced with the PTS1 C-terminal sequence, its path to the peroxisomal matrix is dependent upon binding to another cytosolic protein called pex5 (peroxin 5). Once bound, pex5 interacts with a peroxisomal membrane protein pex14 to form a complex. When the pex5 protein with bound cargo interacts with the pex14 membrane protein, the complex induces the release of the targeted protein into the matrix. Upon releasing the cargo protein into the matrix, pex5 dissociation from pex14 occurs via ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 19 ...
ylation by a membrane complex comprising pex2, pex12, and pex10 followed by an ATP dependent removal involving the cytosolic protein complex pex1 and pex6. The cycle for pex5 mediated import into the peroxisomal matrix is restored after the ATP dependent removal of ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 19 ...
and is free to bind with another protein containing a PTS1 sequence. Proteins containing a PTS2 targeting sequence are mediated by a different cytosolic protein but are believed to follow a similar mechanism to that of those containing the PTS1 sequence.
Diseases
Protein transport is defective in the following genetic diseases: * Mohr–Tranebjaerg syndrome *Zellweger syndrome
Zellweger syndrome is a rare congenital disorder characterized by the reduction or absence of functional peroxisomes in the cells of an individual. It is one of a family of disorders called Zellweger spectrum disorders which are leukodystrophy, l ...
*Adrenoleukodystrophy
Adrenoleukodystrophy (ALD) is a genetic disorder, disease linked to the X chromosome. It is a result of fatty acid buildup caused by failure of peroxisome#Metabolic functions, peroxisomal fatty acid beta oxidation which results in the accumulation ...
(ALD).
* Refsum disease
*Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
*Hypercholesterolemia
Hypercholesterolemia, also called high cholesterol, is the presence of high levels of cholesterol in the blood. It is a form of hyperlipidemia (high levels of lipids in the blood), hyperlipoproteinemia (high levels of lipoproteins in the blood), ...
, atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis, characterized by development of abnormalities called lesions in walls of arteries. This is a chronic inflammatory disease involving many different cell types and is driven by eleva ...
, obesity
Obesity is a medical condition, considered by multiple organizations to be a disease, in which excess Adipose tissue, body fat has accumulated to such an extent that it can potentially have negative effects on health. People are classifi ...
, and diabetes
Diabetes mellitus, commonly known as diabetes, is a group of common endocrine diseases characterized by sustained high blood sugar levels. Diabetes is due to either the pancreas not producing enough of the hormone insulin, or the cells of th ...
In bacteria and archaea
As discussed above (seeprotein translocation
Protein targeting or protein sorting is the Mechanism (biology), biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, diffe ...
), most prokaryotic membrane-bound and secretory proteins are targeted to the plasma membrane by either a co-translation pathway that uses bacterial SRP or a post-translation pathway that requires SecA and SecB. At the plasma membrane, these two pathways deliver proteins to the SecYEG translocon for translocation. Bacteria may have a single plasma membrane (Gram-positive bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
The Gram stain ...
), or an inner membrane plus an outer membrane separated by the periplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in Gram-negative (more accurately "diderm") bacteria. Using cryo-electron micros ...
(Gram-negative bacteria
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the Crystal violet, crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelo ...
). Besides the plasma membrane the majority of prokaryotes lack membrane-bound organelles as found in eukaryotes, but they may assemble proteins onto various types of inclusions such as gas vesicles and storage granules.
Gram-negative bacteria
In gram-negative bacteria proteins may be incorporated into the plasma membrane, the outer membrane, the periplasm or secreted into the environment. Systems for secreting proteins across the bacterial outer membrane may be quite complex and play key roles in pathogenesis. These systems may be described as type I secretion, type II secretion, etc.Gram-positive bacteria
In most gram-positive bacteria, certain proteins are targeted for export across the plasma membrane and subsequent covalent attachment to the bacterial cell wall. A specialized enzyme, sortase, cleaves the target protein at a characteristic recognition site near the protein C-terminus, such as an LPXTG motif (where X can be any amino acid), then transfers the protein onto the cell wall. Several analogous systems are found that likewise feature a signature motif on the extra-cytoplasmic face, a C-terminal transmembrane domain, and cluster of basic residues on the cytosolic face at the protein's extreme C-terminus. The PEP-CTERM/exosortase
Exosortase refers to a family of integral membrane proteins that occur in Gram-negative bacteria that recognizes and cleaves the carboxyl-terminal protein targeting, sorting signal PEP-CTERM. The name derives from a predicted role analogous to so ...
system, found in many Gram-negative bacteria, seems to be related to extracellular polymeric substance
Extracellular polymeric substances (EPS) are biopolymer, natural polymers of molecular mass, high molecular weight secreted by microorganisms into their environment. EPS establish the functional and structural integrity of biofilms, and are consid ...
production. The PGF-CTERM/archaeosortase A system in archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
is related to S-layer
An S-layer (surface layer) is a part of the cell envelope found in almost all archaea, as well as in many types of bacteria.
The S-layers of both archaea and bacteria consists of a Monolayer, monomolecular layer composed of only one (or, in a few c ...
production. The GlyGly-CTERM/rhombosortase system, found in the Shewanella, Vibrio, and a few other genera, seems involved in the release of proteases, nucleases, and other enzymes.
Bioinformatic tools
* Minimotif Miner is a bioinformatics tool that searches protein sequence queries for a known protein targeting sequence motifs.Phobius
predicts signal peptides based on a supplied primary sequence.
SignalP
predicts signal peptide cleavage sites.
LOCtree
predicts the subcellular localization of proteins.
Notes
See also
* Bulk flow *COPI
COPI is a coatomer, a protein complex that coats vesicle (biology), vesicles transporting proteins from the ''cis'' end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally Translation (genetics), synthesi ...
* COPII
The coat protein complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. Thi ...
* Clathrin
Clathrin is a protein that plays a role in the formation of coated vesicles. Clathrin was first isolated by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskel ...
* LocDB
* PSORTdb
* Signal peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16–30 amino acids long) present at the ...
References
External links
* {{DEFAULTSORT:Protein Targeting Post-translational modification Membrane proteins