|
Sorting And Assembly Machinery
The outer mitochondrial membrane is made up of two essential proteins, Tom40 and Sam50. Tom40 Tom40 is a protein import pore required for the import of precursor proteins across the outer mitochondrial membrane, and it makes up part of the translocase of the outer membrane. Sam50 Sam50 is a subunit of the sorting and assembly machinery (SAM) of the outer membrane. The sorting and assembly machinery is a protein complex, that operates after the translocase of the outer membrane, to mediate insertion of beta barrel proteins into the outer mitochondrial membrane. Complex components The sorting and assembly machinery is made up of three subunits, Sam35, Sam37, and Sam50, of which Sam50 is embedded within the outer mitochondrial membrane.Bolender, N., Sickmann, A., Wagner, R., Meisinger, C., Pfanner, N. (2008) Multiple pathways for sorting mitochondrial precursor proteins. EMBO J 9(1): 42-49. Both Sam35 and Sam37 are located on the cytosolic face of the SAM complex are periphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outer Mitochondrial Membrane
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal '' Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number of unicellular organisms, such as microsporidia, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pore
Pore may refer to: Biology Animal biology and microbiology * Sweat pore, an anatomical structure of the skin of humans (and other mammals) used for secretion of sweat * Hair follicle, an anatomical structure of the skin of humans (and other mammals) used for secretion of sebum * Canal pore, an anatomical structure that is part of the lateral line sense system of some aquatic organisms * Gonopore, a genital pore present in some invertebrates, particularly insects * Ozopore, the external discharge site of defensive glands in millipedes and some arachnids * An opening across both inner and outer bacterial membranes, a part of many Gram-negative bacterial secretion systems * One of the openings communicating with the skin surface at the terminus of lactiferous ducts in milk-producing mammals Plant and fungal biology * Germ pore, a small pore in the outer wall of a fungal spore through which the germ tube exits upon germination * Stoma, a small opening on a plant leaf used fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translocase Of The Outer Membrane
The translocase of the outer membrane (TOM) is a complex of proteins found in the outer mitochondrial membrane of the mitochondria. It allows movement of proteins through this barrier and into the intermembrane space of the mitochondrion. Most of the proteins needed for mitochondrial function are encoded by the nucleus of the cell. The outer membrane of the mitochondrion is impermeable to large molecules greater than 5000 daltons. The TOM works in conjunction with the translocase of the inner membrane (TIM) to translocate proteins into the mitochondrion. Many of the proteins in the TOM complex, such as TOMM22, were first identified in '' Neurospora crassa'' and ''Saccharomyces cerevisiae ''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungal microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have be ...''. Many of the genes encoding th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Barrel
In protein structures, a beta barrel (β barrel) is a beta sheet (β sheet) composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are arranged in an antiparallel fashion. Beta barrel structures are named for resemblance to the barrels used to contain liquids. Most of them are water-soluble outer membrane proteins and frequently bind hydrophobic ligands in the barrel center, as in lipocalins. Others span cell membranes and are commonly found in porins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative bacteria. It has been shown that more than 600 proteins with various function such as oxidase, dismutase, and amylase contain the beta barrel structure. In many cases, the strands contain alternating polar and non-polar (hydrophilic and hydrophobic) amino acids, so that the hydrophobic residues are orient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peripheral Membrane Proteins
Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins. The reversible attachment of proteins to biological membranes has shown to regulate cell signaling and many other important cellular events, through a variety of mechanisms. For example, the close association between many en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porin (protein)
Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria (mycolic acid-containing actinomycetes), the outer membrane of mitochondria, and the outer chloroplast membrane (outer plastid membrane). Structure Porins are composed of beta sheets (β sheets) made up of beta strands (β strands) which are linked together by beta turns (β turns) on the cytoplasmic side and long loops of amino acids on the other. The β strands lie in an antiparallel fashion and form a cylindrical tube, called a beta barrel (β barrel). The amino acid composition of the porin β strands are unique in that polar and nonpolar residues alternate along them. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermembrane Space
The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the Inner mitochondrial membrane, inner membrane and the Outer mitochondrial membrane, outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function. Intermembrane space of mitochondria Mitochondria are surrounded by two membranes; the inner and outer mitochondrial membranes. These two membranes allow the formation of two aqueous compartments, which are the intermembrane space (IMS) and the matrix. Channel proteins called porins in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
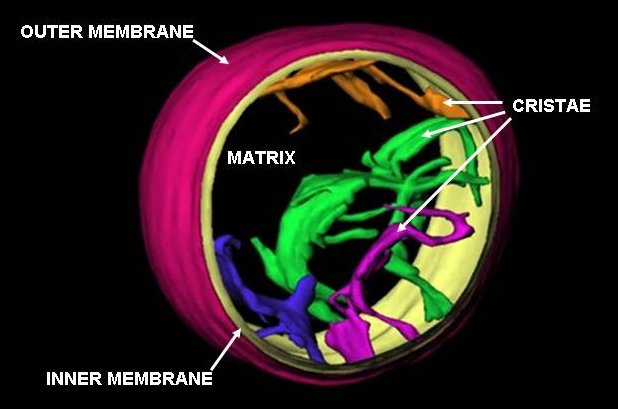
Mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal '' Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number of unicellular organisms, such as microspo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Tim Proteins
Tim9 and Tim10 make up the group of essential small Tim proteins that assist in transport of hydrophobic precursors across the intermembrane space in mammalian cells. Both Tim9 and Tim10 form a hexamer, the Tim9-Tim10 complex, that when associated, functions as a chaperone to assist translocation of preproteins from the outer mitochondrial membrane to the translocase of the inner membrane. The functional Tim9-Tim10 complex not only directs preproteins to the inner mitochondrial membrane in order to interact with the TIM22 complex, but also guides β-barrel precursor proteins to the sorting and assembly machinery (SAM) of the outer membrane. Structure of the Tim9-Tim10 complex The Tim9-Tim10 complex is made up of three Tim9 molecules and three Tim10 molecules. Each Tim9 and Tim10 subunit consists of 80-110 amino acid residues with four conserved cysteine residues that form two intramolecular disulfide bonds. Each subunit folds into a helix-loop-helix structure, with each loop form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
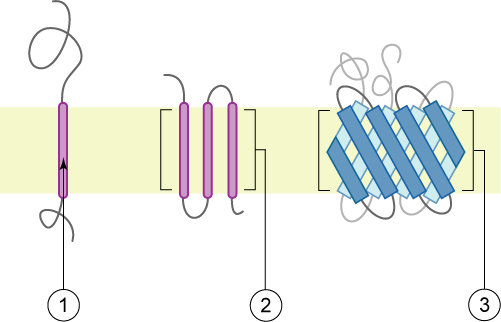
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (Transmembrane protein, transmembrane) or associate with one or the other side of a membrane (Integral monotopic protein, integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct (Native state, native) Protein structure, conformation of the protein in isolation from its native ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |