|
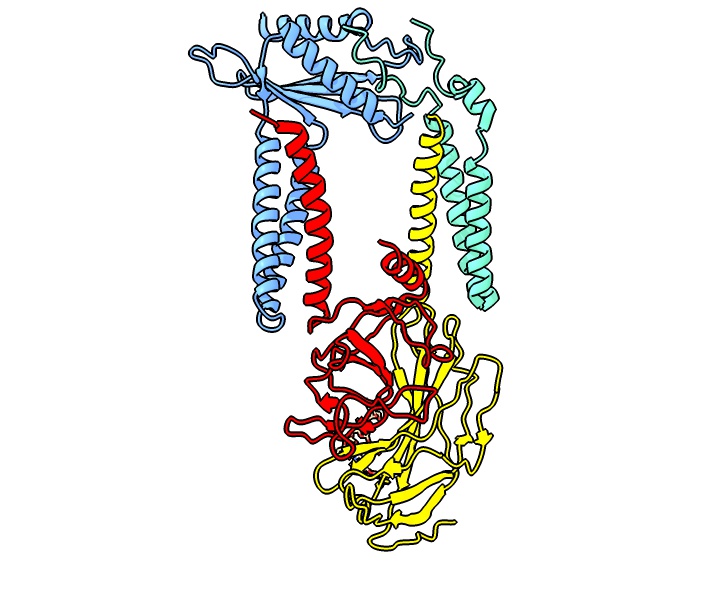
Signal Peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini. Signal peptidases were initially observed in endoplasmic reticulum (ER)-derived membrane fractions isolated from mouse myeloma cells. The key observation by César Milstein and colleagues was that immunoglobulin light chains were produced in a higher molecular weight form, which became processed by the ER membrane fraction. This finding was directly followed by the discovery of the translocation machinery. Signal peptidases are also found in prokaryotes A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ... as well as the protein import machinery of mitochondria and chloroplasts. All signal peptidases described s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16–30 amino acids long) present at the N-terminus (or occasionally nonclassically at the C-terminus or internally) of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles (the endoplasmic reticulum, Golgi or endosomes), secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, most type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide. Function (translocation) Signal peptides function to prompt a cell to transloc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myeloma
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, renal insufficiency, and infections may occur. Complications may include hypercalcemia and amyloidosis. The cause of multiple myeloma is unknown. Risk factors include obesity, radiation exposure, family history, age and certain chemicals. There is an increased risk of multiple myeloma in certain occupations. This is due to the occupational exposure to aromatic hydrocarbon solvents having a role in causation of multiple myeloma. Multiple myeloma is the result of a multi-step malignant transformation, and almost universally originates from the pre-malignant stage monoclonal gammopathy of undetermined significance (MGUS). As MGUS evolves into MM, another pre-stage of the disease is reached, known as smoldering myeloma (SMM). In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
César Milstein
César Milstein, CH, FRS (8 October 1927 – 24 March 2002) was an Argentine biochemist in the field of antibody research. Milstein shared the Nobel Prize in Physiology or Medicine in 1984 with Niels Kaj Jerne and Georges J. F. Köhler for developing the hybridoma technique for the production of monoclonal antibodies. Biography Milstein was born in Bahía Blanca, Argentina. His parents were Máxima (Vanarks) and Lázaro Milstein, a Jewish Ukrainian immigrant. He graduated from the University of Buenos Aires and obtained a PhD under Professor Stopani (Professor of Biochemistry). Later he became a member of the Medical Research Council Laboratory of Molecular Biology, Cambridge, England; he acquired British citizenship and had dual British-Argentinian nationality. In 1956, he received an award from the Sociedad Argentina de Investigation eon Bio Quimica (SAIB) for his work on enzyme kinetics with the enzyme aldehyde dehydrogenase. In 1958, funded by the British Council, he j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translocon
The translocon (also known as a translocator or translocation channel) is a complex of proteins associated with the translocation of polypeptides across membranes. In eukaryotes the term translocon most commonly refers to the complex that transports nascent polypeptides with a targeting signal sequence into the interior (cisternal or lumenal) space of the endoplasmic reticulum (ER) from the cytosol. This translocation process requires the protein to cross a hydrophobic lipid bilayer. The same complex is also used to integrate nascent proteins into the membrane itself (membrane proteins). In prokaryotes, a similar protein complex transports polypeptides across the (inner) plasma membrane or integrates membrane proteins. In either case, the protein complex is formed from Sec proteins (Sec: secretory), with the hetero-trimeric Sec61 being the channel. In prokaryotes, the homologous channel complex is known as SecYEG. This article focuses on the cell's native translocons, but pathogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryotes
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' or 'kernel'. In the earlier two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. However, in the three-domain system, based upon molecular phylogenetics, prokaryotes are divided into two domains: Bacteria and Archaea. A third domain, Eukaryota, consists of organisms with nuclei. Prokaryotes evolved before eukaryotes, and lack nuclei, mitochondria, and most of the other distinct organelles that characterize the eukaryotic cell. Some unicellular prokaryotes, such as cyanobacteria, form colonies held together by biofilms, and large colonies can create multilayered microbial mats. Prokaryotes are asexual, reproducing via binary fission. Horizontal gene transfer is comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal '' Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number of unicellular organisms, such as microspo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroplasts
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which capture the Radiant energy, energy from sunlight and convert it to chemical energy and release oxygen. The chemical energy created is then used to make sugar and other organic molecules from carbon dioxide in a process called the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in some unicellular algae, up to 100 in plants like ''Arabidopsis'' and wheat. Chloroplasts are highly dynamic—they circulate and are moved around within cells. Their behavior is strongly influenced by environmental factors like light color and intensity. Chloroplasts cannot be made anew by the plant cell and must ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Proteases
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like. Classification The MEROPS protease classification system counts 16 superfamilies (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolution of the catalytic mechanism. The majority belong to the S1 family of the PA clan (superfamily) of proteases. For superfamilies, P: superfamily, containing a mixture of nucleophile class families, S: purely serine proteases. superfamily. Within each superfamily, families are designated by their catalytic nucleophile, (S: serine proteases). Substrate specificity Serine prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |