Phosphoenolpyruvate Carboxylase on:
[Wikipedia]
[Google]
[Amazon]
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; , PDB ID: 3ZGE) is an
 # The bicarbonate acts as a
# The bicarbonate acts as a
 PEP carboxylase is mainly subject to two levels of regulation:
PEP carboxylase is mainly subject to two levels of regulation:
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
(HCO3−) to phosphoenolpyruvate
Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP) is the carboxylic acid derived from the enol of pyruvate and a phosphate anion. It exists as an anion. PEP is an important intermediate in biochemistry. It has the high-energy phosphate, highest-e ...
(PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
:
:PEP + HCO3− → oxaloacetate + Pi
This reaction is used for carbon fixation
Biological carbon fixation, or сarbon assimilation, is the Biological process, process by which living organisms convert Total inorganic carbon, inorganic carbon (particularly carbon dioxide, ) to Organic compound, organic compounds. These o ...
in CAM
Cam or CAM may refer to:
Science and technology
* Cam (mechanism), a mechanical linkage which translates motion
* Camshaft, a shaft with a cam
* Camera or webcam, a device that records images or video
In computing
* Computer-aided manufacturin ...
(crassulacean acid metabolism) and organisms, as well as to regulate flux through the citric acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-Co ...
(also known as Krebs or TCA cycle) in bacteria and plants. The enzyme structure and its two step catalytic, irreversible mechanism have been well studied. PEP carboxylase is highly regulated, both by phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
and allostery.
Enzyme structure
The PEP carboxylase enzyme is present in plants and some types of bacteria, but not in fungi or animals (including humans). The genes vary between organisms, but are strictly conserved around the active and allosteric sites discussed in the mechanism and regulation sections.Tertiary structure
Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the ...
of the enzyme is also conserved.
The crystal structure of PEP carboxylase in multiple organisms, including ''Zea mays'' (maize), and ''Escherichia coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly fo ...
'' has been determined. The overall enzyme exists as a dimer-of-dimers: two identical subunits closely interact to form a dimer through salt bridges between arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) a ...
(R438 - exact positions may vary depending on the origin of the gene) and glutamic acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can ...
(E433) residues. This dimer assembles (more loosely) with another of its kind to form the four subunit complex. The monomer subunits are mainly composed of alpha helices (65%), and have a mass of 106kDa each. The sequence length is about 966 amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s.;
The enzyme active site is not completely characterized. It includes a conserved aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protei ...
(D564) and a glutamic acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can ...
(E566) residue that non-covalently bind a divalent metal cofactor ion through the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
functional groups. This metal ion can be magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
, manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
or cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
depending on the organism, and its role is to coordinate the phosphoenolpyruvate molecule as well as the reaction intermediates. A histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
(H138) residue at the active site is believed to facilitate proton transfer during the catalytic mechanism.
Enzyme mechanism
The mechanism of PEP carboxylase has been well studied. The enzymatic mechanism of forming oxaloacetate is very exergonic, and thereby irreversible, in biochemical standard conditions; the biological standardGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
change (∆G°’) is −30 kJ⋅mol−1. The substrates and cofactor bind in the following order: metal cofactor (either Co2+, Mg2+, or Mn2+), PEP, bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
(HCO3−). The mechanism proceeds in two major steps, as described below and shown in figure 2:
 # The bicarbonate acts as a
# The bicarbonate acts as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
to attack the phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
group in PEP. This results in the splitting of PEP into a carboxyphosphate and the (very reactive) enolate form of pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
.
# Proton transfer takes place at the carboxyphosphate. This is most likely modulated by a histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
(H138) residue that first deprotonates the carboxy side, and then, as an acid, protonates the phosphate part. The carboxyphosphate then exothermically decomposes into carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and inorganic phosphate, at this point making this an irreversible reaction. Finally, after the decomposition, the carbon dioxide is attacked by the enolate to form oxaloacetate.
The metal cofactor is necessary to coordinate the enolate and carbon dioxide intermediates; the CO2 molecule is only lost 3% of the time. The active site is hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
to exclude water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, since the carboxyphosphate intermediate is susceptible to hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
.
Function
The three most important roles that PEP carboxylase plays in plants and bacteria metabolism are in the cycle, the CAM cycle, and thecitric acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-Co ...
biosynthesis flux.
The primary mechanism of carbon dioxide assimilation in plants is through the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (also known as RuBisCO), that adds CO2 to ribulose-1,5-bisphosphate (a 5 carbon sugar), to form two molecules of 3-phosphoglycerate (2x3 carbon sugars). However, at higher temperatures and lower CO2 concentrations, RuBisCO adds oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
instead of carbon dioxide, to form the unusable product glycolate in a process called photorespiration. To prevent this wasteful process, plants increase the local CO2 concentration in a process called the cycle. PEP carboxylase plays the key role of binding CO2 in the form of bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
with PEP to create oxaloacetate in the mesophyll tissue. This is then converted back to pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
(through a malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
intermediate), to release the CO2 in the deeper layer of bundle sheath cells for carbon fixation by RuBisCO and the Calvin cycle. Pyruvate is converted back to PEP in the mesophyll cells, and the cycle begins again, thus actively pumping CO2.
The second important and very similar biological significance of PEP carboxylase is in the CAM cycle. This cycle is common in organisms living in arid habitats. Plants cannot afford to open stomata
In botany, a stoma (: stomata, from Greek ''στόμα'', "mouth"), also called a stomate (: stomates), is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange between the internal air spa ...
during the day to take in CO2, as they would lose too much water by transpiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. It is a passive process that requires no energy expense by the plant. Transpiration also cools plants, c ...
. Instead, stomata open at night, when water evaporation is minimal, and take in CO2 by fixing with PEP to form oxaloacetate though PEP carboxylase. Oxaloacetate is converted to malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
by malate dehydrogenase, and stored for use during the day when the light dependent reaction generates energy (mainly in the form of ATP) and reducing equivalents such as NADPH to run the Calvin cycle.
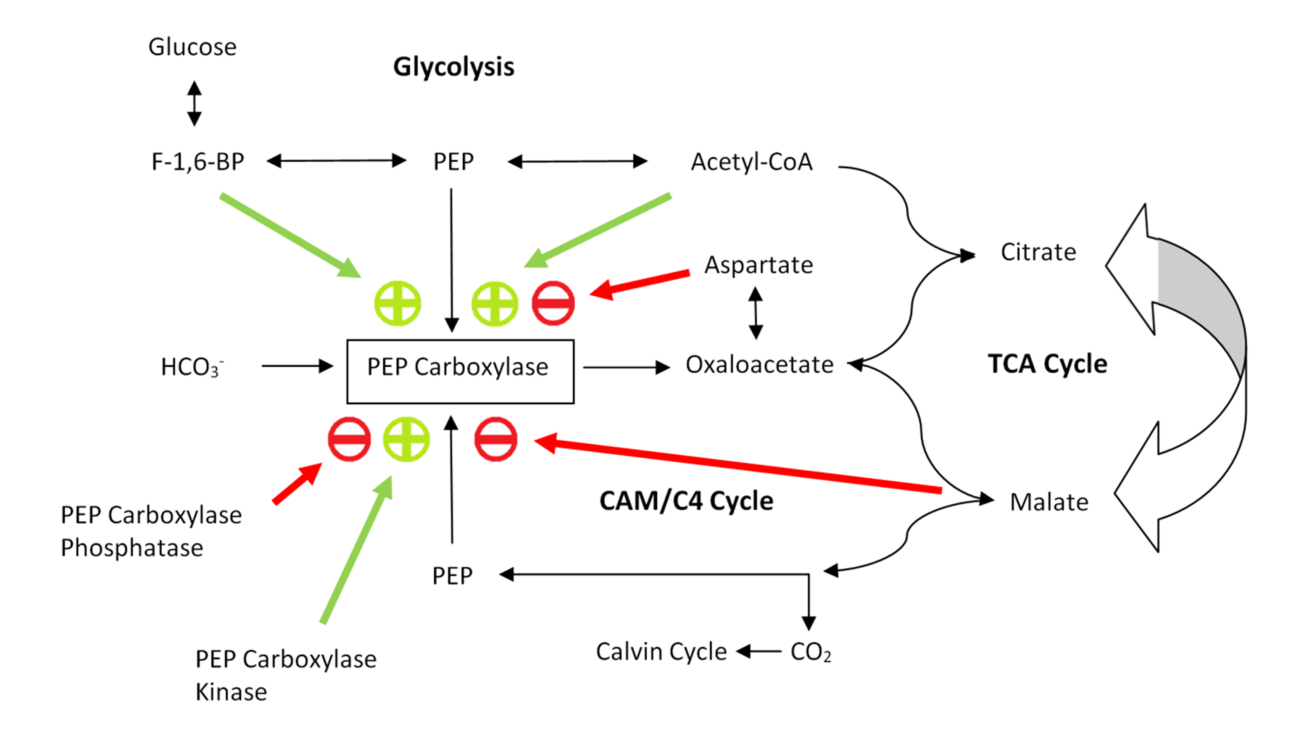
Third, PEP carboxylase is significant in non-photosynthetic metabolic pathways. Figure 3 shows this metabolic flow (and its regulation). Similar to pyruvate carboxylase, PEP carboxylase replenishes oxaloacetate in the citric acid cycle. At the end of glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
, PEP is converted to pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
, which is converted to acetyl-coenzyme-A (acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, o ...
), which enters the citric acid cycle by reacting with oxaloacetate to form citrate. To increase flux through the cycle, some of the PEP is converted to oxaloacetate by PEP carboxylase. Since the citric acid cycle intermediates provide a hub for metabolism, increasing flux is important for the biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
of many molecules, such as for example amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
.
Regulation
 PEP carboxylase is mainly subject to two levels of regulation:
PEP carboxylase is mainly subject to two levels of regulation: phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
and allostery. Figure 3 shows a schematic of the regulatory mechanism.
Phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
by phosphoenolpyruvate carboxylase kinase turns the enzyme on, whereas phosphoenolpyruvate carboxylase phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate ...
turns it back off. Both kinase and phosphatase are regulated by transcription. It is further believed that malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
acts as a feedback inhibitor of kinase expression levels, and as an activator for phosphatase expression (transcription). Since oxaloacetate is converted to malate in CAM and organisms, high concentrations of malate activate phosphatase expression - the phosphatase subsequently de-phosphorylates and thus de-actives PEP carboxylase, leading to no further accumulation of oxaloacetate and thus no further conversion of oxaloacetate to malate. Hence malate production is down-regulated.
The main allosteric inhibitors of PEP carboxylase are the carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
(weak) and aspartate (strong). Since malate is formed in the next step of the CAM and cycles after PEP carboxylase catalyses the condensation of CO2 and PEP to oxaloacetate, this works as a feedback inhibition pathway. Oxaloacetate and aspartate are easily inter-convertible through a transaminase mechanism; thus high concentrations of aspartate are also a pathway of feedback inhibition of PEP carboxylase.
The main allosteric activators of PEP carboxylase are acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, o ...
and fructose-1,6-bisphosphate (F-1,6-BP). Both molecules are indicators of increased glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
levels, and thus positive feed-forward effectors of PEP carboxylase. They signal the need to produce oxaloacetate to allow more flux through the citric acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-Co ...
. Additionally, increased glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
means a higher supply of PEP is available, and thus more storage capacity for binding CO2 in transport to the Calvin cycle. It is also noteworthy that the negative effectors aspartate competes with the positive effector acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, o ...
, suggesting that they share an allosteric binding site.
Studies have shown that energy equivalents such as AMP, ADP and ATP have no significant effect on PEP carboxylase.
The magnitudes of the allosteric effects of these different molecules on PEP carboxylase activity depend on individual organisms.
References
{{Portal bar, Biology, border=no EC 4.1.1 Photosynthesis