A phosphetane is a 4-membered
organophosphorus
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective in ...
heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
. The parent phosphetane molecule, which has the formula C
3H
7P, is one atom larger than
phosphirane
Phosphirane is the organophosphorus compound with the formula C2H4PH. It is a colorless gas of no commercial value. As the simplest cyclic, saturated organophosphorus compound, phosphirane is the prototype of a family of related compounds that ha ...
s, one smaller than
phosphole
Phosphole is the organic compound with the chemical formula ; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve a ...
s, and is the heavy-atom analogue of
azetidine
Azetidine is a Saturated and unsaturated compounds, saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to mo ...
s. The first known phosphetane synthesis was reported in 1957 by Kosolapoff and Struck,
but the method was both inefficient and hard to reproduce, with yields rarely exceeding 1%. A far more efficient method was reported in 1962 by McBride,
whose method allowed for the first studies into the physical and chemical properties of phosphetanes. Phosphetanes are a well understood class of molecules that have found broad applications as chemical building blocks, reagents for organic/inorganic synthesis, and ligands in coordination chemistry.
Synthesis
Many methods towards the synthesis of phosphetanes have been developed since 1957. The following are the most utilized.
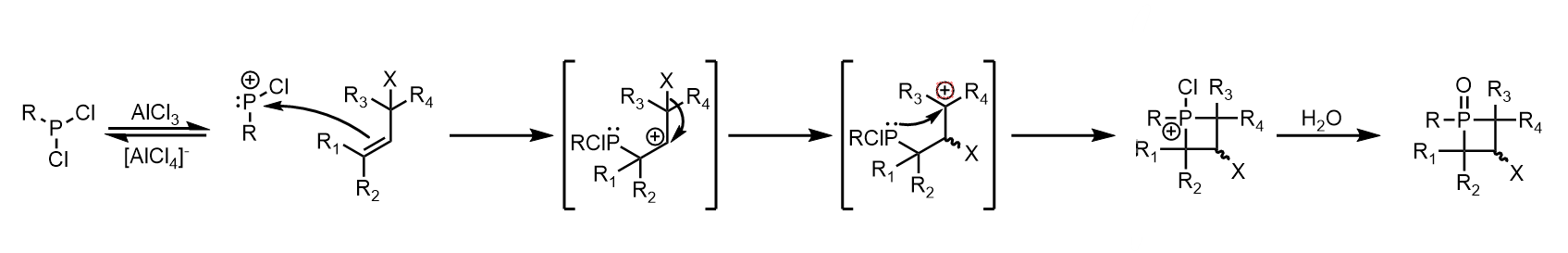
McBride method (Electrophilic addition to olefins)
The method initially outlined by McBride has been developed for singular
alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, as well as dienes. Both types follow the same general mechanism: formation of a
phosphenium
Phosphenium ions, not to be confused with phosphonium or phosphirenium, are divalent cations of phosphorus of the form R2sup>+. Phosphenium ions have long been proposed as reaction intermediates.
Synthesis Legacy methods
The first cyclic phos ...
cation from a dichlorophosphine and aluminum trichloride,
electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Chem ...
by an alkene to the phosphenium,
carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
rearrangement, intramolecular
nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions ...
of the new alkyl
phosphine
Phosphine ( IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotti ...
to the carbocation, and oxidation of the resulting
phosphetanium with water to obtain a
phosphetane oxide. Limitations of this approach are unpredictable carbocation rearrangement in more complexly branched alkanes, the incompatibility of carbocations with many
nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
functional groups, and the risk of cation quenching by
elimination
Elimination may refer to:
Science and medicine
* Elimination reaction, an organic reaction in which two functional groups split to form an organic product
*Bodily waste elimination, discharging feces, urine, or foreign substances from the bo ...
pathways.
Mono-ene addition
In the case of
electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that car ...
addition by a single alkene,
carbocation rearrangement occurs via
hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
or alkyl shifts. The general scheme for phosphetane synthesis from mono-enes is given below:
Diene addition
In the case of electrophilic addition by a diene, carbocation rearrangement first occurs via cation-π cyclization. The general scheme for phosphetane synthesis from dienes is given below:

Alkylation and intramolecular cyclization
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
and cyclization pathways have been developed for both phosphines and phosphine oxides.
From phosphines
The synthesis of phosphetanes from P(III) alkylation and subsequent cyclization usually proceeds through sequential
phosphanide
Phosphanides are chemicals containing the H2sup>− anion. This is also known as the phosphino anion or phosphido ligand. The IUPAC name can also be dihydridophosphate(1−).
It can occur as a group phosphanyl -PH2 in organic compounds or ligand ...
/phosphine displacement of 1,3-alkyl
dihalides or
sulfonate esters
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, n ...
(OTf, OTs, OMs, etc.).
The phosphanide source is commonly the lithium salt, but can also be accessed by in situ deprotonation of phosphines. The
SN2 mechanism associated with this transformation comes with the advantage of
stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Co ...
, but at the expense of electrophilic or epimerizable functional group tolerance and kinetically slow reactivity with secondary/tertiary leaving groups. The general mechanism is seen below:

From phosphine oxides
Similar syntheses from P(V) compounds are known but are far rarer due to their relative inefficiency and unpredictability.
This preparation features the in situ formation of a
Grignard reagent, followed by intramolecular addition/cyclization to a phosphine oxide, all on an n-propyl backbone. This was the method employed by Kosolapoff and Struck in the first synthesis of a phosphetane. The general mechanism is seen below:

Cyclopropane ring-expansion
Another way to make phosphetanes comes from the
ring-expansion of
cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself i ...
s, in which it seems a phosphine is directly inserted into a C-C bond. The true mechanism of this transformation is similar to that of the McBride synthesis and is sometimes classified as such, with similar advantages and drawbacks. Although relieving the cyclopropane
ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are s ...
is of great assistance in the initial C-P bond, exhaustive alkyl substitution to stabilize the formed carbocation is often required. The general mechanism is seen below:

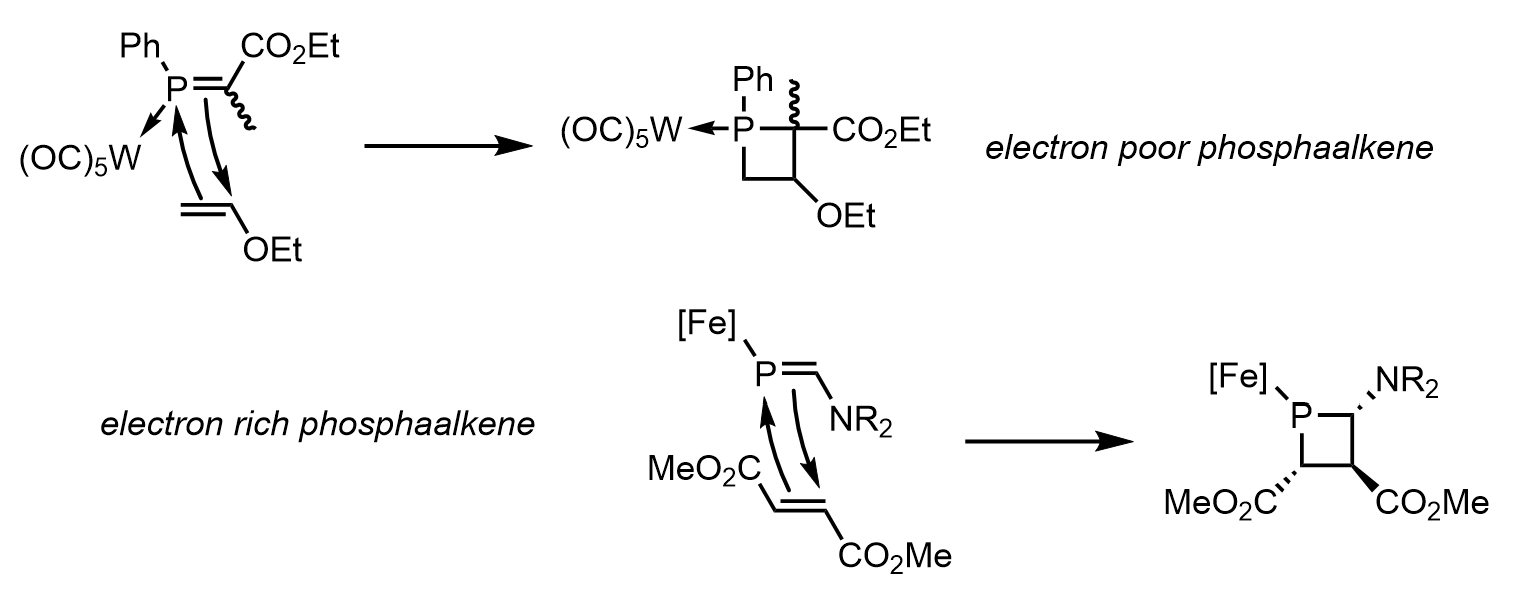
+2cycloaddition
One final method that has been observed to produce phosphetanes is the
+2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". ...
of
phosphaalkene
Phosphaalkenes ( IUPAC name: alkylidenephosphanes) are organophosphorus compounds with double bonds between carbon and phosphorus(III) with the formula R2C=PR. In the compound phosphorine one carbon atom in benzene is replaced by phosphorus. The ...
s and olefins. This method is not often discussed for its tendency to produce phosphetanes, but rather for its insight into the reactivity of the much more elusive phosphaalkenes. The difficult synthesis of these phosphaalkenes severely limits the utility of the method as it relates to phosphetane synthesis, despite its attractive stereospecific and modular approach. This usually involves a Lewis acid bound phosphorus, and can occur with electron rich phosphaalkenes and electron poor olefins,
or the inverse. An example of each, and the mechanism, are seen below:
Structure and bonding
Experimental and
crystallographic
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wo ...
data exists for many of the phosphetane types listed below, however, all of the geometric and electronic (
HOMO and LUMO) information below was determined
theoretically with the B3LYP
functional
Functional may refer to:
* Movements in architecture:
** Functionalism (architecture)
** Form follows function
* Functional group, combination of atoms within molecules
* Medical conditions without currently visible organic basis:
** Functional s ...
and DEF2-SVP
basis set using ORCA (5.0.3) for the parent molecule at each
coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
to provide a general and consistent trend as an introduction to the subject. Geometries and orbital plots were generated using Avogadro (4.1).
Dicoordinate phosphetanes
Though rarely reported in the literature, if at all, dicoordinate phosphetanes of phosphenium, phosphanide, and phosphorus radical archetypes are theoretically possible as transient reactive intermediates. Their optimized physical and electronic geometries are presented mainly as a means of comparison to the more commonly observed tri, tetra, and pentacoordinate phosphetanes.
Phosphenium ion
The phosphenium case is
isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
to a cyclic
carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" may ...
. The optimized geometry is quite planar in comparison to the other dicoordinate cases, with its HOMO and LUMO being the exocyclic
lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
and empty p-orbital, respectively.
File:Phosphenium.png, Optimized Geometry
File:P+homo.png, HOMO
File:P+lumo.png, LUMO
Phosphorus radical
The optimized geometry and frontier molecular orbitals for the dicoordinate phosphorus radical are similar to the phosphenium case. The ring is slightly less planar, and the HOMO is now a singly occupied p-orbital. The lone pair is the HOMO-1.
File:PhosphaRadical.png, Optimized Geometry
File:P.homo.png, HOMO
File:P.lumo.png, LUMO
Phosphanide ion
The phosphanide case is isoelectronic to cyclic
ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
. In this ion, there is significantly more pucker within the phosphetane ring, along with widening of the C-P-C angle, but the HOMO and HOMO-1 are similar to the radical case, now both being doubly occupied.
File:Phosphanide.png, Optimized Geometry
File:P-homo.png, HOMO
File:P-lumo.png, LUMO
Tricoordinate phosphetanes
Tricoordinate phosphetanes are well known in the literature and exemplify the classical trigonal pyramidal P(III) phosphorus center.
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mo ...
is introduced in these tricoordinate molecules, albeit with a very low kinetic barrier (~2.45 kcal/mol for the given example),
in which the hydrogen can be pseudo-axial (as shown), or pseudo-equatorial. The pseudo-axial conformer is the more stable of the two. Since the lone pair is larger, it settles in the pseudo-equatorial position, but this is inverted rather swiftly due to minimization of steric clash as R becomes bigger than H. The phosphetane ring is puckered, not planar, due to the asymmetry above and below the ring about phosphorus. As is expected, the HOMO is the nucleophilic lone pair usually associated with phosphines.
File:PHPhosphine.png, Optimized Geometry
File:PHhomo.png, HOMO
File:PHlumo.png, LUMO
Tetracoordinate phosphetanes
Tetracoordinate phosphetanes are by far the most commonly observed geometry around the phosphorus center, usually as the ubiquitous P(V) phosphorus oxide center, but not uncommonly as phosphetanium ions.
Phosphetanium ion
The phosphetanium is isoelectronic to a tetracoordinate carbon and assumes its tetrahedral geometry, greatly planarizing the ring by increasing molecular symmetry. Deviation from this would occur with any change of one of the hydrogen atoms with a bulkier group, after which, the ring would pucker, with the larger substituent pseudo-equatorial. The
acidity
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
of the α-carbon hydrogens is significantly increased due to the charge neutralization driving force; this is reflected in the C-H σ-antibonding contributions to the LUMO.
File:PHH+Phosphetanium.png, Optimized Geometry
File:PHH+homo.png, HOMO
File:PHH+lumo.png, LUMO
Phosphetane oxide
The other classic phosphorus compound is the tetrahedral P(V) phosphine oxide. Like tricoordinate phosphetanes, phosphetane oxides also exhibit isomerism, this time with a much larger kinetic barrier. When the oxide is pseudo-equatorial (as shown), the designation is ''trans'', while when the oxide is pseudo-axial, the compound is ''cis''. The preference for one over the other is largely based on the middle carbon substitution, rather than the oxide. As one may expect of a covalently bound oxide, the HOMO is an oxygen lone pair and the LUMO is largely contributed to by the P-O π-antibonding interaction.
File:PHOPhosphetaneOxide.png, Optimized Geometry
File:PHOhomo.png, HOMO
File:PHOlumo.png, LUMO
Pentacoordinate phosphetanes
Pentacoordinate phosphetanes, or phosphoranes, present an alternative geometric mantle on which a P(V) phosphorus center may exist. It is important to note that this class of phosphoranes are typically not trigonal bipyramidal, but closer to square pyramidal. A result of this geometric perturbation is the emergence of a P-H σ-antibonding that is represented prominently in the LUMO, accounting for the characteristic Lewis acidity of square pyramidal phosphoranes.
File:PHHHPhosphorane.png, Optimized Geometry
File:PHHHhomo.png, HOMO
File:PHHHlumo.png, LUMO
Hexacoordinate phosphetanes
Hexacoordinate, anionic phosphates are mainly known in the literature as counterions (
hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this an ...
), but are theoretically possible as reactive intermediates for associative mechanisms at phosphorus centers. In this compound, phosphorus assumes the expected octahedral geometry. As expected for this hexacoordinate phosphate, C-H σ-bonding orbitals comprise the HOMO, accounting for the expected
hydricity due to favorable charge neutralization. Similar to the dicoordinate case, these optimized physical and electronic geometries are presented mainly as a means of comparison to the more commonly observed tri, tetra, and pentacoordinate phosphetanes.
File:PHHHH-Hexacoordinate.png, Optimized Geometry
File:PHHHH-homo.png, HOMO
File:PHHHH-lumo.png, LUMO
Reactivity
Phoshetanes display a broad range of reactivity and appear in the literature in many different facets of a chemical reaction. There are cases where phosphetanes themselves are the substrate of interest, cases where phosphetanes are observed as transient intermediates during a chemical reaction, cases where phosphetanes are used as the active
reagents in chemical reactions, and cases where phosphetanes are ligated to a metal that is the active reagent in a given process. All of these overarching scenarios are discussed in more detail below.
Inherent reactivity
Much of the reactivity inherent to, or performed directly on, phosphetane substrates is an ode to its ring strain, calculated to be ~17.9 kcal/mol.
The release of some or all of this strain energy drive the two characteristic types of reactivity observed: ring expansion and ring opening. Reactivity at the phosphorus center, including
reduction, oxidation, and phosphorane formation as well as alkylation of ring carbons can be performed without cleavage of the ring in some instances, representing the final types of inherent reactivity. These four will be discussed in more detail below.
Ring opening reactions
Phosphetane ring opening reactions have been of synthetic interest in the past as a potential method for the creation of polypropylphosphine
polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
s and materials, but despite ring opening reactions occurring, the
polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of such material has only been sparsely observed in very concentrated solutions.
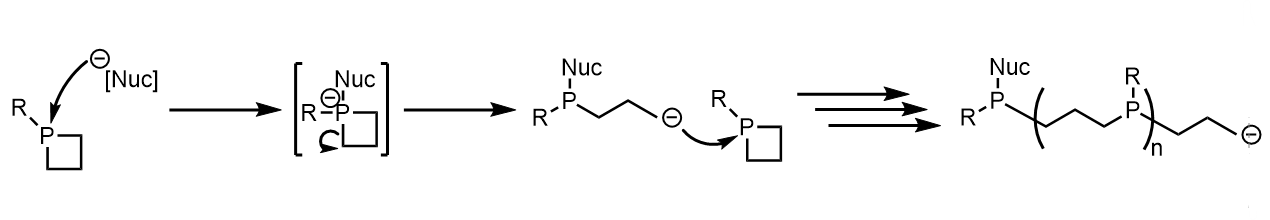
The main observation of ring opening is as a byproduct of other intended reactions, such as phosphetanium oxidation
and α-carbon functionalization.

One intentional and constructive method of ring-opening has been outlined in the literature and features a phosphetane
ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ato ...
that undergoes Wittig reactivity with
aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s to form γ-unsaturated phosphine oxides.

Ring expansion reactions
Methods of ring expansion to insert carbon, oxygen, and nitrogen atoms into phosphetane rings to produce the corresponding phospholes exist but are of limited synthetic utility due to their unpredictable stereo and regioselectivity on unsymmetric phosphetanes. Insertion of carbon typically involves the addition of water to a phosphetanium featuring a leaving group
or pi-system
(usually
enones but also phenyl groups) alpha to phosphorus that is liberated by alkyl migration after collapse of the phosphetane oxide.

Insertion of oxygen into the P-C bond of a phosphetane oxide is done with
mCPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mC ...
and proceeds via a currently unknown mechanism with unusually high regioselectivity for the less substituted carbon.

Nitrogen atom insertion proceeds from photolysis of an azidophosphetane oxide, presumably from a
Curtius type rearrangement from the generated
nitrene
In chemistry, a nitrene or imene () is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore cons ...
. Though this is the proposed mechanism, there are clear doubts about the N=P=O intermediate.

Reactivity at phosphorus
Redox between P(III) phosphetanes and P(V) phosphetane oxides are possible and well documented through the use of mild reagents such as oxygen or water and silicon hydrides to achieve oxidation and reduction, respectively.

More interesting is the synthesis of stable 5-coordinate phosphetanes (
phosphoranes
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the hypothetical molecule PH5. The derivative pentaphenylphosp ...
) from both traditional P(III) phosphines and P(V) phosphine oxides, in addition to P(V) phosphetanium ions, via a couple general methods. With respect to phosphine substrates, phosphorane synthesis usually occurs via reaction with
peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
s/
disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s or
perfluoro
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound containing only carbon-fluorines and C−C bonds, as well as potentially heteroatoms. Perfluorinated compounds have properties that result from the presence of flu ...
π-systems, such as perfluoro acetone, for which the mechanism is unresolved, or perfluoro 1,3-butadiene.

Methods to access phosphoranes from P(V) oxides and phosphetaniums are usually through stepwise
deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to org ...
-nucleophilic addition pathways, or direct addition pathways,
respectively. Nucleophiles are usually halides or alkoxy functional groups, and in the case of deoxygenation-substitution, the two nucleophiles can be either tethered (e.g.
catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
) or not.

α-Carbon functionalization
The final portion of inherent reactivity of phosphetanes to be discussed is the functionalization of the phosphetane oxide alpha carbons, almost always through deprotonation with organolithium reagents, followed by S
N2 displacement of an alkyl halide. The use of chiral axillaries on phosphorus can make this process stereoselective.

Reactive intermediates
The appearance of phosphetanes and derivatives thereof is well documented in organic chemistry literature as reactive intermediates for a myriad of different processes. These processes include, but are not limited to,
Wittig Wittig is a surname, and may refer to:
* Burghardt Wittig (born 1947), German biochemist
* Curt Wittig, American chemist
* David Wittig (born 1955), American executive
* Edward Wittig (1879–1941), Polish sculptor
* Ferdinand Wittig (1851-1909), ...
,
Horner-Wadsworth-Emmons,
Corey-Fuchs, and
Seyferth-Gilbert chemistries. All of these processes include the in-situ formation and decomposition of
oxaphosphetane
An oxaphosphetane is a molecule containing a four-membered ring with one phosphorus, one oxygen and two carbon atoms. In a 1,2-oxaphosphetane phosphorus is bonded directly to oxygen, whereas a 1,3-oxaphosphetane has the phosphorus and oxygen atom ...
intermediates through
metathesis-type pathways to form alkenes or
alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
s from aldehydes and a phosphorus reagent.
Reagents and catalysts
Since the early 2010s, much progress has been made in the development of phosphetanes as useful reagents and
catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
to complement
transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
catalysts in organic synthesis. These efforts have primarily been made by the research group of Dr. Alexander Radosevich at
Pennsylvania State University
The Pennsylvania State University (Penn State or PSU) is a public state-related land-grant research university with campuses and facilities throughout Pennsylvania. Founded in 1855 as the Farmers' High School of Pennsylvania, Penn State becam ...
, and subsequently the
Massachusetts Institute of Technology
The Massachusetts Institute of Technology (MIT) is a Private university, private Land-grant university, land-grant research university in Cambridge, Massachusetts. Established in 1861, MIT has played a key role in the development of modern t ...
, but contributions from the lab of Dr. Thomas Werner at the Leibniz-Institut für Katalyse (Leibniz Institute for Catalysis) have also been impactful. The common theme underpinning these works is an active phosphetane species reductively acting on a substrate, resulting in formation of phosphetane oxide and the desired product, followed by reduction of the phosphetane oxide back to the phosphetane with a mild silicon hydride which closes the catalytic cycle.

The uncharacteristic biphilic nature of these phosphines, and other
non-trigonal pnictogen compounds, is a result of
molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain ...
perturbation, in this case, imposed by the ring strain inherent to phospetanes. Most of these transformations are probed based on
stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
reactivity of the phosphetane, illustrating their utility as catalysts or reagents in the event there is substrate incompatibility with the hydride. Below is the general catalytic cycle and an abbreviated list of reactions that can be catalyzed through this method.

Ligands for transition metal complexes
Transition metal complexes with ligated P(III) phosphetanes are known for tungsten, iron,
molybdenum,
platinum,
ruthenium,
rhodium,
palladium,
iridium,
and possibly more, to produce
achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
, racemic, and optically pure coordination complexes. Despite these efforts, the intricate details about their nature as
ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s and effects on metal centers as it deviates from traditional phosphines is relatively understudied. Direct comparison of classic bis-trialkylphosphinedichloroplatinum(II) complexes with the corresponding phosphetane containing complex possibly enumerate a weakened σ-
trans effect In inorganic chemistry, the trans effect is the increased lability of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands. It is attributed to electronic effects and it is most notable in square p ...
and
π-accepting character of the phosphetane ligand, most likely due to the aforementioned symmetry distortion, corroborated by short Pt-P (2.208 and 2.210 angstrom) and Pt-Cl (2.342 and 2.355 angstrom) bonds.
More work is needed to make this claim categorically.
Most of the study and interest in phosphetanes as ligands is there ability to impart enantioselectivity on certain catalytic
hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
, reduction,
and π-allyl
reactions when using the corresponding chiral phosphetanes. As is the case for most asymmetric catalysis, disfavored
steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
interaction between chiral ligands, substrate, and other reagents are credited for the observed enantio or diastereoselectivity, though it seems the use of more traditional chiral phosphines has proved more popular than that of chiral phosphetanes. Below are select examples of enantioselective catalysis using phosphetane ligands.

References
{{Reflist
Phosphorus heterocycles
Four-membered rings






 The main observation of ring opening is as a byproduct of other intended reactions, such as phosphetanium oxidation and α-carbon functionalization.
The main observation of ring opening is as a byproduct of other intended reactions, such as phosphetanium oxidation and α-carbon functionalization.
 One intentional and constructive method of ring-opening has been outlined in the literature and features a phosphetane
One intentional and constructive method of ring-opening has been outlined in the literature and features a phosphetane 
 Insertion of oxygen into the P-C bond of a phosphetane oxide is done with
Insertion of oxygen into the P-C bond of a phosphetane oxide is done with  Nitrogen atom insertion proceeds from photolysis of an azidophosphetane oxide, presumably from a Curtius type rearrangement from the generated
Nitrogen atom insertion proceeds from photolysis of an azidophosphetane oxide, presumably from a Curtius type rearrangement from the generated 
 More interesting is the synthesis of stable 5-coordinate phosphetanes (
More interesting is the synthesis of stable 5-coordinate phosphetanes ( Methods to access phosphoranes from P(V) oxides and phosphetaniums are usually through stepwise
Methods to access phosphoranes from P(V) oxides and phosphetaniums are usually through stepwise 

 The uncharacteristic biphilic nature of these phosphines, and other non-trigonal pnictogen compounds, is a result of
The uncharacteristic biphilic nature of these phosphines, and other non-trigonal pnictogen compounds, is a result of 
