peptide synthesis on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 SPPS is limited by reaction yields due to the exponential accumulation of by-products, and typically peptides and proteins in the range of 70 amino acids are pushing the limits of synthetic accessibility. Synthetic difficulty also is sequence dependent; typically aggregation-prone sequences such as
SPPS is limited by reaction yields due to the exponential accumulation of by-products, and typically peptides and proteins in the range of 70 amino acids are pushing the limits of synthetic accessibility. Synthetic difficulty also is sequence dependent; typically aggregation-prone sequences such as
 Carbodiimides such as dicyclohexylcarbodiimide (DCC) and
Carbodiimides such as dicyclohexylcarbodiimide (DCC) and 

 Carbodiimide activation opens the possibility for racemization of the activated amino acid. Racemization can be circumvented with 'racemization suppressing' additives such as the
Carbodiimide activation opens the possibility for racemization of the activated amino acid. Racemization can be circumvented with 'racemization suppressing' additives such as the
 To avoid epimerization through the O-acylisourea intermediate formed when using a carbodiimide reagent, an amidinium- or
To avoid epimerization through the O-acylisourea intermediate formed when using a carbodiimide reagent, an amidinium- or
 Before the advent of SPPS, solution methods for chemical peptide synthesis relied on ''tert''-butyloxycarbonyl (abbreviated 'Boc') as a temporary N-terminal α-amino protecting group. The Boc group is removed with acid, such as
Before the advent of SPPS, solution methods for chemical peptide synthesis relied on ''tert''-butyloxycarbonyl (abbreviated 'Boc') as a temporary N-terminal α-amino protecting group. The Boc group is removed with acid, such as
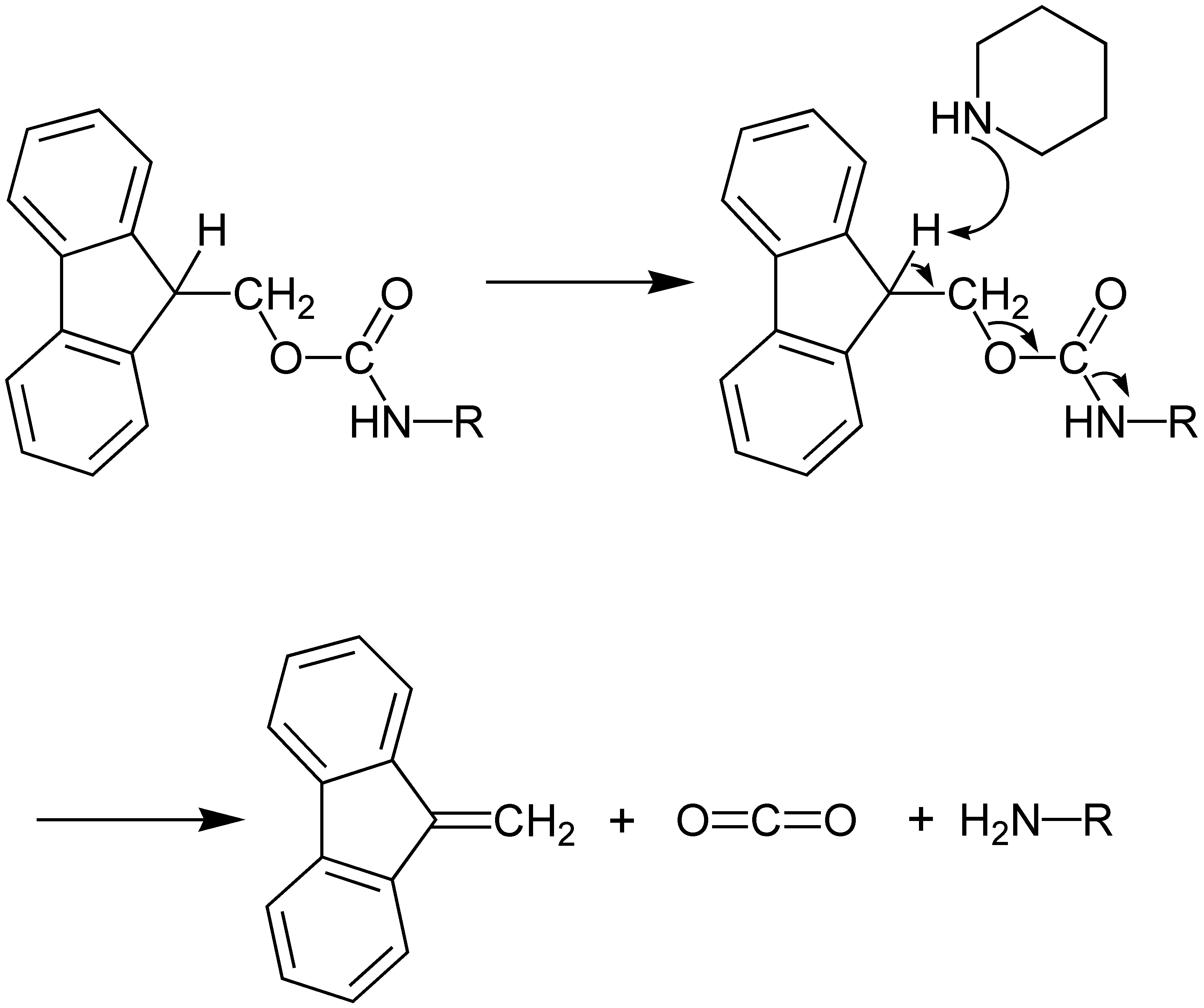 The use of N-terminal Fmoc protection allows for a milder deprotection scheme than used for Boc/Bzl SPPS, and this protection scheme is truly orthogonal under SPPS conditions. Fmoc deprotection utilizes a base, typically 20–50%
The use of N-terminal Fmoc protection allows for a milder deprotection scheme than used for Boc/Bzl SPPS, and this protection scheme is truly orthogonal under SPPS conditions. Fmoc deprotection utilizes a base, typically 20–50%
 It is removed under harsh conditions using HBr in
It is removed under harsh conditions using HBr in
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, peptide synthesis is the production of peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...
s, compounds where multiple amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s are linked via amide bonds, also known as peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s. Peptides are chemically synthesized by the condensation reaction of the carboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
of one amino acid to the amino group of another. Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
). Protein biosynthesis
Protein biosynthesis, or protein synthesis, is a core biological process, occurring inside Cell (biology), cells, homeostasis, balancing the loss of cellular proteins (via Proteolysis, degradation or Protein targeting, export) through the produc ...
(long peptides) in living organisms occurs in the opposite direction.
The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below). Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes moreover.
Although recombinant protein is more cost effective for large-scale production, chemical synthesis facilitates the production of peptides that are difficult to express
Express, The Expresss or EXPRESS may refer to:
Arts, entertainment and media Film
* ''Express: Aisle to Glory'', a 1998 comedy short film featuring Kal Penn
* ''The Express: The Ernie Davis Story'', a 2008 film starring Dennis Quaid
* The Expre ...
in bacteria, the incorporation of unnatural amino acids, peptide/protein backbone modification, and the synthesis of D-proteins, which consist of D-amino acids.
Solid-phase synthesis
The established method for the production of synthetic peptides in the lab is known as solid phase peptide synthesis (SPPS). Pioneered by Robert Bruce Merrifield, SPPS allows the rapid assembly of a peptide chain through successive reactions of amino acid derivatives on a macroscopically insoluble solvent-swollen beaded resin support.Characteristics of solid support
The solid support consists of small, polymeric resin beads functionalized with reactive groups (such as amine or hydroxyl groups) that link to the nascent peptide chain. Since the peptide remains covalently attached to the support throughout the synthesis, excess reagents and side products can be removed by washing and filtration. This approach circumvents the comparatively time-consuming isolation of the product peptide from solution after each reaction step, which would be required when using conventional solution-phase synthesis.General mechanism
Each amino acid to be coupled to the peptide chain N-terminus must be protected on its N-terminus and side chain using appropriate protecting groups such as Boc (acid-labile) or Fmoc (base-labile), depending on the side chain and the protection strategy used (see below). The general SPPS procedure is one of repeated cycles of alternate N-terminal deprotection and coupling reactions. The resin can be washed between each steps. Reactions in SPPS are conducted as follows: # The amine group of the first amino acid is protected with Fmoc or Boc group # Protected amino acid is coupled with free amino groups attached to resin beads # Protecting group is removed (see: Protecting groups schemes) # The second amino acid with a ''N''-protecting group is coupled with the first one. Coupling reagents are employed to help the formation of the peptide bond. # The above cycle is repeated until the desired sequence has been synthesised # Optionally, the ''N''-terminal amino group undergoes capping, thereby preventing the obtained peptide from further reaction # The crude product is purified using either: #* reverse-phase high-performance liquid chromatography (HPLC) #* multicolumn countercurrent solvent gradient purification (MCSGP) which is utilised mainly in the case of longer peptides, due to accumulation of numerous minor byproducts that have similar properties to the desired peptide product. This process is used to maximise the yield without sacrificing purity. SPPS is limited by reaction yields due to the exponential accumulation of by-products, and typically peptides and proteins in the range of 70 amino acids are pushing the limits of synthetic accessibility. Synthetic difficulty also is sequence dependent; typically aggregation-prone sequences such as
SPPS is limited by reaction yields due to the exponential accumulation of by-products, and typically peptides and proteins in the range of 70 amino acids are pushing the limits of synthetic accessibility. Synthetic difficulty also is sequence dependent; typically aggregation-prone sequences such as amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human ...
s are difficult to make. Longer lengths can be accessed by using ligation approaches such as native chemical ligation Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most ...
, where two shorter fully deprotected synthetic peptides can be joined in solution.
Peptide coupling reagents
An important feature that has enabled the broad application of SPPS is the generation of extremely high yields in the coupling step. Highly efficientamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
bond-formation conditions are required. To illustrate the impact of suboptimal coupling yields for a given synthesis, consider the case where each coupling step were to have at least 99% yield: this would result in a 77% overall crude yield for a 26-amino acid peptide (assuming 100% yield in each deprotection); if each coupling were 95% efficient, the overall yield would be 25%. and adding an excess of each amino acid (between 2- and 10-fold). The minimization of amino acid racemization during coupling is also of vital importance to avoid epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is t ...
ization in the final peptide product.
Amide bond formation between an amine and carboxylic acid is slow, and as such usually requires 'coupling reagents' or 'activators'. A wide range of coupling reagents exist, due in part to their varying effectiveness for particular couplings, many of these reagents are commercially available.
Carbodiimides
diisopropylcarbodiimide
''N'','-Diisopropylcarbodiimide is a carbodiimide used in peptide synthesis. As a liquid, it is easier to handle than the commonly used Dicyclohexylcarbodiimide, ''N'','-dicyclohexylcarbodiimide, a waxy solid. In addition, ''N'','-diisopropylurea ...
(DIC) are frequently used for amide bond formation. The reaction proceeds via the formation of a highly reactive ''O''-acylisourea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
. This reactive intermediate is attacked by the peptide N-terminal amine, forming a peptide bond. Formation of the ''O''-acylisourea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
proceeds fastest in non-polar solvents such as dichloromethane.
DIC is particularly useful for SPPS since as a liquid it is easily dispensed, and the urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
byproduct is easily washed away. Conversely, the related carbodiimide 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) is often used for solution-phase peptide couplings as its urea byproduct can be removed by washing during aqueous work-up.

 Carbodiimide activation opens the possibility for racemization of the activated amino acid. Racemization can be circumvented with 'racemization suppressing' additives such as the
Carbodiimide activation opens the possibility for racemization of the activated amino acid. Racemization can be circumvented with 'racemization suppressing' additives such as the triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms w ...
s 1-hydroxy-benzotriazole (HOBt), and 1-hydroxy-7-aza-benzotriazole (HOAt). These reagents attack the ''O''-acylisourea intermediate to form an active ester In organic chemistry, an active ester is an ester functional group that is highly susceptible toward nucleophilic attack. Activation can be imparted by modifications of the acyl or the alkoxy components of a normal ester, say ethyl acetate. Typical ...
, which subsequently reacts with the peptide to form the desired peptide bond. Ethyl cyanohydroxyiminoacetate (Oxyma), an additive for carbodiimide coupling, acts as an alternative to HOAt.
Amidinium and phosphonium salts
 To avoid epimerization through the O-acylisourea intermediate formed when using a carbodiimide reagent, an amidinium- or
To avoid epimerization through the O-acylisourea intermediate formed when using a carbodiimide reagent, an amidinium- or phosphonium
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The ...
-reagent can be employed These reagents have two parts: an electrophilic moiety which deoxygenates the carboxylic acid (blue) and masked nucleophilic moiety (red). Nucleophilic attack of the carboxylic acid on the electrophilic amidinium or phosphonium moiety leads to a short lived intermediate which is rapidly trapped by the unmasked nucleophile to form the activated ester intermediate and either a urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
or phosphoramide by-product. These cationic reagents have non-coordinating counteranions such as a hexafluorophosphate
Hexafluorophosphate is an fluoroanion, anion with chemical formula of . It is an Octahedral molecular geometry, octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the Hexafluorosilicic acid, h ...
or a tetrafluoroborate. The identity of this anion is typically indicated by the first letter in the reagent’s acronym, although the nomenclature can be inconsistent. For example HBTU is a hexafluorophosphate salt while TBTU is a tetrafluoroborate salt. In addition to HBTU and HATU other common reagents include HCTU (6-ClHOBt), TCFH (chloride) and COMU (ethyl cyano(hydroxyimino)acetate). Amidinium reagents incorporating hydroxybenzotriazole moieties can exist in an N-form (guanadinium) or an O-form (uronium), but the N-form is generally more stable. Phosphonium reagents include BOP (HOBt), PyBOP (HOBt) and PyAOP (HOAt). Although these reagents can lead to the same activated ester intermediates as a carbodiimide reagent, the rate of activation is higher due to the high electrophilicty of these cationic reagents. Amidinium reagents are capable of reacting with the peptide N-terminus to form an inactive guanidino by-product, whereas phosphonium reagents are not.Propanephosphonic acid anhydride
Since late 2000s, propanephosphonic acid anhydride, sold commercially under various names such as "T3P", has become a useful reagent for amide bond formation in commercial applications. It converts the oxygen of the carboxylic acid into a leaving group, whose peptide-coupling byproducts are water-soluble and can be easily washed away. In a performance comparison between propanephosphonic acid anhydride and other peptide coupling reagents for the preparation of a nonapeptide drug, it was found that this reagent was superior to other reagents with regards to yield and low epimerization.Solid supports
Solid supports for peptide synthesis are selected for physical stability, to permit the rapid filtration of liquids. Suitable supports are inert to reagents and solvents used during SPPS and allow for the attachment of the first amino acid. Swelling is of great importance because peptide synthesis takes place inside the swollen pores of the solid support. Three primary types of solid supports are: gel-type supports, surface-type supports, and composites. Improvements to solid supports used for peptide synthesis enhance their ability to withstand the repeated use of TFA during the deprotection step of SPPS. Two primary resins are used, based on whether a C-terminal carboxylic acid or amide is desired. The Wang resin was, , the most commonly used resin for peptides with C-terminal carboxylic acids.Protecting groups schemes
As described above, the use of N-terminal and side chainprotecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep synthesis, multistep organic ...
is essential during peptide synthesis to avoid undesirable side reactions, such as self-coupling of the activated amino acid leading to (polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
). This would compete with the intended peptide coupling reaction, resulting in low yield or even complete failure to synthesize the desired peptide.
Two principle protecting group schemes are typically used in solid phase peptide synthesis: so-called Boc/benzyl and Fmoc/''tert-''butyl approaches. The Boc/Bzl strategy utilizes TFA-labile N-terminal Boc protection alongside side chain protection that is removed using anhydrous hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
during the final cleavage step (with simultaneous cleavage of the peptide from the solid support). Fmoc/tBu SPPS uses base-labile Fmoc N-terminal protection, with side chain protection and a resin linkage that are acid-labile (final acidic cleavage is carried out via TFA treatment). Both approaches, including the advantages and disadvantages of each, are outlined in more detail below.
Boc/Bzl SPPS
trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
(TFA). This forms a positively charged amino group in the presence of excess TFA (note that the amino group is not protonated in the image on the right), which is neutralized and coupled to the incoming activated amino acid. Neutralization can either occur prior to coupling or ''in situ'' during the basic coupling reaction.
The Boc/Bzl approach retains its usefulness in reducing peptide aggregation during synthesis. In addition, Boc/benzyl SPPS may be preferred over the Fmoc/''tert-''butyl approach when synthesizing peptides containing base-sensitive moieties (such as depsipeptides or thioester moeities), as treatment with base is required during the Fmoc deprotection step (see below).
Permanent side-chain protecting groups used during Boc/benzyl SPPS are typically benzyl or benzyl-based groups. Final removal of the peptide from the solid support occurs simultaneously with side chain deprotection using anhydrous hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
via hydrolytic cleavage. The final product is a fluoride salt which is relatively easy to solubilize. Scavengers such as cresol
Cresols (also known as hydroxytoluene, toluenol, benzol or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also c ...
must be added to the HF in order to prevent reactive cations from generating undesired byproducts.
Fmoc/''t''Bu SPPS
 The use of N-terminal Fmoc protection allows for a milder deprotection scheme than used for Boc/Bzl SPPS, and this protection scheme is truly orthogonal under SPPS conditions. Fmoc deprotection utilizes a base, typically 20–50%
The use of N-terminal Fmoc protection allows for a milder deprotection scheme than used for Boc/Bzl SPPS, and this protection scheme is truly orthogonal under SPPS conditions. Fmoc deprotection utilizes a base, typically 20–50% piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor de ...
in DMF. The exposed amine is therefore neutral, and consequently no neutralization of the peptide-resin is required, as in the case of the Boc/Bzl approach. The lack of electrostatic repulsion between the peptide chains can lead to increased risk of aggregation with Fmoc/''t''Bu SPPS however. Because the liberated fluorenyl group is a chromophore, Fmoc deprotection can be monitored by UV absorbance of the reaction mixture, a strategy which is employed in automated peptide synthesizers.
The ability of the Fmoc group to be cleaved under relatively mild basic conditions while being stable to acid allows the use of side chain protecting groups such as Boc and ''t''Bu that can be removed in milder acidic final cleavage conditions (TFA) than those used for final cleavage in Boc/Bzl SPPS (HF). Scavengers such as water and triisopropylsilane (TIPS) are most commonly added during the final cleavage in order to prevent side reactions with reactive cationic species released as a result of side chain deprotection. Nevertheless, many other scavenger compounds could be used as well. The resulting crude peptide is obtained as a TFA salt, which is potentially more difficult to solubilize than the fluoride salts generated in Boc SPPS.
Fmoc/''t''Bu SPPS is less atom-economical, as the fluorenyl group is much larger than the Boc group. Accordingly, prices for Fmoc amino acids were high until the large-scale piloting of one of the first synthesized peptide drugs, enfuvirtide
Enfuvirtide (International Nonproprietary Name, INN), sold under the brand name Fuzeon, is an HIV fusion inhibitor, the first of a class of antiretroviral drugs used in combination therapy for the treatment of AIDS/HIV.
Medical uses
Enfuvirti ...
, began in the 1990s, when market demand adjusted the relative prices of Fmoc- vs Boc- amino acids.
Other protecting groups
=Benzyloxy-carbonyl
= The (Z) group is another carbamate-type amine protecting group, discovered byLeonidas Zervas
Leonidas Zervas (, ; 21 May 1902 – 10 July 1980) was a Greeks, Greek Organic chemistry, organic chemist who made seminal contributions in Peptide synthesis, peptide chemical synthesis. Together with his mentor Max Bergmann they laid the founda ...
in the early 1930s and usually added via reaction with benzyl chloroformate.
acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, or milder conditions of catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
.
This methodology was first used in the synthesis of oligopeptides by Zervas and Max Bergmann in 1932. Hence, this became known as the Bergmann-Zervas synthesis, which was characterised "epoch-making" and helped establish synthetic peptide chemistry as a distinct field. It constituted the first useful lab method for controlled peptide synthesis, enabling the synthesis of previously unattainable peptides with reactive side-chains, while Z-protected amino acids are also prevented form undergoing racemization.
The use of the Bergmann-Zervas method remained the standard practice in peptide chemistry for two full decades after its publication, superseded by newer methods (such as the Boc protecting group) in the early 1950s. Nowadays, while it has been used periodically for α-amine protection, it is much more commonly used for side chain protection.
=Alloc and miscellaneous groups
= The allyloxycarbonyl (alloc) protecting group is sometimes used to protect an amino group (or carboxylic acid or alcohol group) when an orthogonal deprotection scheme is required. It is also sometimes used when conducting on-resin cyclic peptide formation, where the peptide is linked to the resin by a side-chain functional group. The Alloc group can be removed usingtetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon d ...
.
For special applications like synthetic steps involving protein microarray
A protein microarray (or protein chip) is a high-throughput method used to track the interactions and activities of proteins, and to determine their function, and determining function on a large scale. Its main advantage lies in the fact that larg ...
s, protecting groups sometimes termed "lithographic" are used, which are amenable to photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 Nanometre, nm), visible ligh ...
at a particular wavelength of light, and so which can be removed during lithographic
Lithography () is a planographic method of printing originally based on the immiscibility of oil and water. The printing is from a stone (lithographic limestone) or a metal plate with a smooth surface. It was invented in 1796 by the German ...
types of operations.
Regioselective disulfide bond formation
The formation of multiple native disulfides remains challenging of native peptide synthesis by solid-phase methods. Random chain combination typically results in several products with nonnative disulfide bonds. Stepwise formation of disulfide bonds is typically the preferred method, and performed with thiol protecting groups. Different thiol protecting groups provide multiple dimensions of orthogonal protection. These orthogonally protected cysteines are incorporated during the solid-phase synthesis of the peptide. Successive removal of these groups, to allow for selective exposure of free thiol groups, leads to disulfide formation in a stepwise manner. The order of removal of the groups must be considered so that only one group is removed at a time. Thiol protecting groups used in peptide synthesis requiring later regioselective disulfide bond formation must possess multiple characteristics. First, they must be reversible with conditions that do not affect the unprotected side chains. Second, the protecting group must be able to withstand the conditions of solid-phase synthesis. Third, the removal of the thiol protecting group must be such that it leaves intact other thiol protecting groups, if orthogonal protection is desired. That is, the removal of PG A should not affect PG B. Some of the thiol protecting groups commonly used include the acetamidomethyl (Acm), ''tert''-butyl (But), 3-nitro-2-pyridine sulfenyl (NPYS), 2-pyridine-sulfenyl (Pyr), and trityl (Trt) groups. Importantly, the NPYS group can replace the Acm PG to yield an activated thiol. Using this method, Kiso and coworkers reported the first total synthesis of insulin in 1993. In this work, the A-chain of insulin was prepared with following protecting groups in place on its cysteines: CysA6(But), CysA7(Acm), and CysA11(But), leaving CysA20 unprotected.Microwave-assisted peptide synthesis
Microwave-assisted peptide synthesis has been used to complete long peptide sequences with high degrees of yield and low degrees of racemization.Continuous flow solid-phase peptide synthesis
The first article relating to continuous flow peptide synthesis was published in 1986, but due to technical limitations, it was not until the early 2010's when more academic groups started using continuous flow for the rapid synthesis of peptides. The advantages of continuous flow over traditional batch methods is the ability to heat reagents with good temperature control, allowing the speed of reaction kinetics while minimising side reactions. cycles times vary from 30 seconds, up to 6 minutes, depending on reaction conditions and excess of reagent. Thanks to inline analytics, such as UV/Vis spectroscopy and the use of Variable Bed Flow reactor (VBFR) that monitor the resin volume, on-resin aggregation can be identified and coupling efficiency can be evaluated.Synthesizing long peptides
Stepwise elongation, in which the amino acids are connected step-by-step in turn, is ideal for small peptides containing between 2 and 100 amino acid residues. Another method is fragment condensation, in which peptide fragments are coupled. Although the former can elongate the peptide chain without racemization, the yield drops if only it is used in the creation of long or highly polar peptides. Fragment condensation is better than stepwise elongation for synthesizing sophisticated long peptides, but its use must be restricted in order to protect against racemization. Fragment condensation is also undesirable since the coupled fragment must be in gross excess, which may be a limitation depending on the length of the fragment. A new development for producing longer peptide chains is chemical ligation: unprotected peptide chains react chemoselectively in aqueous solution. A first kinetically controlled product rearranges to form the amide bond. The most common form ofnative chemical ligation Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most ...
uses a peptide thioester that reacts with a terminal cysteine residue.
Other methods applicable for covalently linking polypeptides in aqueous solution include the use of split inteins, spontaneous isopeptide bond formation and sortase ligation.
In order to optimize synthesis of long peptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
, a method was developed in Medicon Valley
Medicon Valley is a leading international life sciences, life-sciences cluster in Europe, spanning the Øresund Region of eastern Denmark and southern Sweden. It is one of Europe's strongest life science clusters, with many life science companies a ...
for converting peptide sequences. The simple pre-sequence (e.g. Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
(Lysn); Glutamic Acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can ...
(Glun); (LysGlu)n) that is incorporated at the C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein
Proteins are large biomolecules and macromolecules that comp ...
of the peptide to induce an alpha-helix-like structure. This can potentially increase biological half-life
Biological half-life (elimination half-life, pharmacological half-life) is the time taken for concentration of a drug, biological substance (such as a medication) to decrease from its maximum concentration (chemistry), concentration (Cmax (pharm ...
, improve peptide stability and inhibit enzymatic degradation without altering pharmacological activity or profile of action.
Cyclic peptides
On resin cyclization
Peptides can be cyclized on a solid support. A variety of cyclization reagents can be used such as HBTU/HOBt/DIEA, PyBop/DIEA, PyClock/DIEA. Head-to-tail peptides can be made on the solid support. The deprotection of the C-terminus at some suitable point allows on-resin cyclization by amide bond formation with the deprotected N-terminus. Once cyclization has taken place, the peptide is cleaved from resin by acidolysis and purified. The strategy for the solid-phase synthesis of cyclic peptides is not limited to attachment through Asp, Glu or Lys side chains. Cysteine has a very reactive sulfhydryl group on its side chain. A disulfide bridge is created when a sulfur atom from one Cysteine forms a single covalent bond with another sulfur atom from a second cysteine in a different part of the protein. These bridges help to stabilize proteins, especially those secreted from cells. Some researchers use modified cysteines using S-acetomidomethyl (Acm) to block the formation of the disulfide bond but preserve the cysteine and the protein's original primary structure.Off-resin cyclization
Off-resin cyclization is a solid-phase synthesis of key intermediates, followed by the key cyclization in solution phase, the final deprotection of any masked side chains is also carried out in solution phase. This has the disadvantages that the efficiencies of solid-phase synthesis are lost in the solution phase steps, that purification from by-products, reagents and unconverted material is required, and that undesiredoligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s can be formed if macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
formation is involved.
The use of pentafluorophenyl esters (FDPP, PFPOH) and BOP-Cl are useful for cyclising peptides.
History
The first protected peptide was synthesised by Theodor Curtius in 1882 and the first free peptide was synthesised byEmil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and List of Nobel laureates in Chemistry, 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fisch ...
in 1901.
See also
* Oligonucleotide synthesis * Clicked peptide polymer * Bailey peptide synthesisReferences
Further reading
* * * * * * * *External links
{{DEFAULTSORT:Peptide Synthesis Chemical synthesis Peptides Peptide coupling reagents Biochemistry methods Biochemistry Amide synthesis reactions