|
Dicyclohexylcarbodiimide
''N'',''N''′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water. Structure and spectroscopy The C−N=C=N−C core of carbodiimides (N=C=N) is linear, being related to the structure of allene. The molecule has idealized C2 symmetry. The N=C=N moiety gives characteristic IR spectroscopic signature at 2117 cm−1. The 15N NMR spectrum shows a characteristic shift of 275 ppm upfield of nitric acid and the 13C NMR spectrum features a peak at about 139 ppm downfield from TMS. Preparation DCC is produced by the decarboxylation of cyclohexylisocyanate using phosphine oxides as a catalyst: :2 C6H11NC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time. Structure and bonding From the perspective of bonding, carbodiimides are isoelectronic with carbon dioxide. Three principal resonance structures describe carbodiimides: :RN=C=NR ↔ RN+≡C-N−R ↔ RN−-C≡N+R The N=C=N core is relatively linear and the C-N=C angles approach 120°. In the case of C(NCHPh2)2, the central N=C=N angle is 170° and the C-N=C angles are within 1° of 126°. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steglich Esterification
The Steglich esterification is a variation of an esterification with dicyclohexylcarbodiimide as a coupling reagent and 4-dimethylaminopyridine as a catalyst. The reaction was first described by Wolfgang Steglich in 1978. It is an adaptation of an older method for the formation of amides by means of DCC (dicyclohexylcarbodiimide) and 1-hydroxybenzotriazole (HOBT). : This reaction generally takes place at room temperature. A suitable solvent is dichloromethane. Because the reaction is mild, esters can be obtained that are inaccessible through other methods for instance esters of the sensitive 2,4-dihydroxybenzoic acid. A characteristic is the formal uptake of water generated in the reaction by DCC, forming the urea compound dicyclohexylurea (DCU). Reaction mechanism The reaction mechanism is described as follows: With amines, the reaction proceeds without problems to the corresponding amides because amines are more nucleophilic In chemistry, a nucleophile is a chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyclohexylurea
Dicyclohexylurea is an organic compound, specifically, a urea. It is the byproduct of the reaction of dicyclohexylcarbodiimide with amines or alcohols. It may be prepared by the reaction of cyclohexylamine Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, ... and ''S'',''S''-dimethyl dithiocarbonate. 1,3-Dicyclohexyl urea (DCU) is a potent soluble epoxide hydrolase (sEH) inhibitor. It has been shown to lower systemic blood pressure by 22 ± 4 mmHg in SHR.{{cite journal , doi = 10.1111/j.1742-7843.2008.00213.x , author = Sarbani Ghosh, Po-Chang Chiang, Jan L. Wahlstrom, Hideji Fujiwara, Jon G. Selbo andSteven L. Roberds , title = Oral Delivery of 1,3-Dicyclohexylurea Nanosuspension Enhances Exposure and Lowers Blood Pressure in Hypertensive Rats , year = 2008, journal = Basic & Cli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Synthesis
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis (long peptides) in living organisms occurs in the opposite direction. The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below). Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N'-Diisopropylcarbodiimide
''N'',''N′''-Diisopropylcarbodiimide is a carbodiimide used in peptide synthesis. As a liquid, it is easier to handle than the commonly used ''N'',''N′''-dicyclohexylcarbodiimide, a waxy solid. In addition, ''N'',''N′''-diisopropylurea, its byproduct in many chemical reactions, is soluble in most organic solvents, a property that facilitates work-up In chemistry, work-up refers to the series of manipulations required to isolate and Chemical purity, purify the Product (chemistry), product(s) of a chemical reaction. Typically, these manipulations may include: * quenching a reaction to deactiva .... Further reading * * {{organic-compound-stub Peptide coupling reagents Carbodiimides Reagents for biochemistry Biochemistry Biochemistry methods Isopropyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
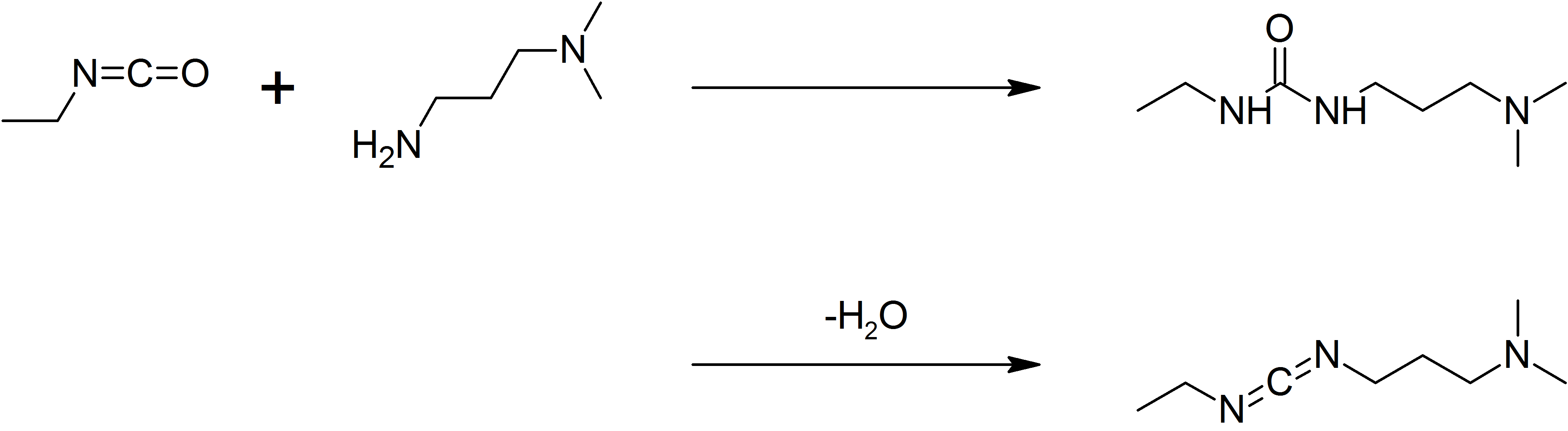
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, EDAC or EDCI) is a water-soluble carbodiimide usually handled as the hydrochloride. It is typically employed in the 4.0-6.0 pH range. It is generally used as a carboxyl activating agent for the coupling of primary amines to yield amide bonds. While other carbodiimides like dicyclohexylcarbodiimide (DCC) or diisopropylcarbodiimide (DIC) are also employed for this purpose, EDC has the advantage that the urea byproduct formed (often challenging to remove in the case of DCC or DIC) can be washed away from the amide product using dilute acid. Additionally, EDC can also be used to activate phosphate groups in order to form phosphomonoesters and phosphodiesters. Common uses for this carbodiimide include peptide synthesis, protein crosslinking to nucleic acids, but also in the preparation of immunoconjugates. EDC is often used in combination with ''N''-hydroxysuccinimide (NHS) for the immobilisation of large biomolecules. Recent wor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Alcohol
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walden Inversion
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in an SN2 reaction, Walden inversion occurs at a tetrahedral carbon atom. It can be visualized by imagining an umbrella turned inside-out in a gale. In the Walden inversion, the backside attack by the nucleophile in an SN2 reaction gives rise to a product whose configuration is opposite to the reactant. Therefore, during SN2 reaction, 100% inversion of product takes place. This is known as Walden inversion. It was first observed by chemist Paul Walden in 1896. He was able to convert one enantiomer of a chemical compound into the other enantiomer and back again in a so-called Walden cycle which went like this: (+) chlorosuccinic acid (1 in the illustration) was converted to (+) malic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium Acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions. Structure With a 1:2 stoichiometric ratio of palladium atoms and acetate ligands, the compound exists as molecular and polymeric forms with the trimeric form being the dominant form in the solid state and in solution. Pd achieves approximate square planar coordination in both forms. As prepared by Geoffrey Wilkinson and coworkers in 1965 and later characterized by Skapski and Smart in 1970 by single crystal X-ray diffraction, palladium(II) acetate is a red-brown solid that crystallizes as monoclinic plates. It has a trimeric structure, consisting of an equilateral triangle of Pd atoms each pair of which is bridged with two acetate groups in a butterfly conforma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |