carbodiimide on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 From the perspective of bonding, carbodiimides are
From the perspective of bonding, carbodiimides are
 Polycarbodiimides can also be used as crosslinkers for aqueous resins, such as polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea. The result is the formation of covalent bonds between the polymer chains, making them crosslinked.
Polycarbodiimides can also be used as crosslinkers for aqueous resins, such as polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea. The result is the formation of covalent bonds between the polymer chains, making them crosslinked.
 DCC (acronym for ''N'',''N''-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for
DCC (acronym for ''N'',''N''-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for
 In contrast to DCC, DIC ( ''N'',''N''-diisopropylcarbodiimide) is a liquid. Its hydrolysis product, N,N'-diisopropylurea, is soluble in organic solvents.
In contrast to DCC, DIC ( ''N'',''N''-diisopropylcarbodiimide) is a liquid. Its hydrolysis product, N,N'-diisopropylurea, is soluble in organic solvents.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
with the formula RN=C=NR. On Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.
A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
in the presence of water over time.
Structure and bonding
 From the perspective of bonding, carbodiimides are
From the perspective of bonding, carbodiimides are isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
. Three principal resonance structures describe carbodiimides:
:RN=C=NR ↔ RN+≡C-N−R ↔ RN−-C≡N+R
The N=C=N core is relatively linear and the C-N=C angles approach 120°. In the case of C(NCHPh2)2, the central N=C=N angle is 170° and the C-N=C angles are within 1° of 126°. The C=N distances are short, nearly 120 pm, as is characteristic of double bonds. Carbodiimides are chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
, possessing C2-symmetry and therefore axial chirality
In chemistry, axial chirality is a special case of chirality (chemistry), chirality in which a molecule contains two pairs of chemical groups in a non-planar arrangement about an axis of chirality so that the molecule is not superposable on its mi ...
. However, due to the low energy barrier to the molecule rotating and thereby converting quickly between its isomers, the actual isolation of one optical isomer of a carbodiimide is extremely difficult. It has been demonstrated at least once, in the case of conformationally restricted cyclic carbodiimides; though there are other reports of one-handed axially chiral carbodiimides, their validity has since been called into question on experimental and computational grounds.
The parent compound, methanediimine, (HN=C=NH), is a tautomer of cyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
.
Synthesis
From thioureas and ureas
A classic route to carbodiimides involves dehydrosulfurization ofthiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), with the oxygen atom replaced by sulfur atom (as implied by the '' thio-'' prefix). The properties of urea and thiourea differ s ...
s. A typical reagent for this process is mercuric oxide:
:(R(H)N)2CS + HgO → (RN)2C + HgS + H2O
This reaction can often be conducted as stated, even though carbodiimides react with water. In some cases, a dehydrating agent is added to the reaction mixture.
The dehydration of N,N'-dialkylureas gives carbodiimides:
:(R(H)N)2CO → (RN)2C + H2O
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula Phosphorus, P4Oxygen, O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desic ...
and p- Toluenesulfonyl chloride have been used as a dehydrating agents.
From isocyanates
Isocyanates can be converted to carbodiimides with loss of carbon dioxide: :2 RN=C=O → (RN)2C + CO2 The reaction is catalyzed byphosphine oxide
Phosphine oxide is the inorganic compound with the formula H3PO. Although stable as a dilute gas, liquid or solid samples are unstable. Unlike many other compounds of the type POxHy, H3PO is rarely discussed and is not even mentioned in major so ...
s. This reaction is reversible.
Reactions
Compared to other heteroallenes, carbodiimides are very weak electrophiles and only react with nucleophiles in the presence of catalysts, such as acids. In this way,guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experi ...
s can be prepared. As weak bases, carbodiimides bind to Lewis acids to give adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
s.
Moffatt oxidation
Carbodiimides are reagents for the Moffatt oxidation, a protocol for conversion of an alcohol to a carbonyl (ketone or aldehyde) usingdimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
as the oxidizing agent:
:(CH3)2SO + (CyN)2C + R2CHOH → (CH3)2S + (CyNH)2CO + R2C=O
Typically the sulfoxide and diimide are used in excess. The reaction generates dimethyl sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula . It is the simplest thioether and has a characteristic disagreeable odor. It is a flammable liquid that boils at . It is a component of the smell produc ...
and a urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
as byproducts.
Coupling agents
Inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, compounds containing the carbodiimide functionality are used as dehydration agents. Specifically they are often used to convert carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s to amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s or ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s. Additives, such as N-hydroxybenzotriazole or N-hydroxysuccinimide, are often added to increase yields and decrease side reactions.
 Polycarbodiimides can also be used as crosslinkers for aqueous resins, such as polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea. The result is the formation of covalent bonds between the polymer chains, making them crosslinked.
Polycarbodiimides can also be used as crosslinkers for aqueous resins, such as polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea. The result is the formation of covalent bonds between the polymer chains, making them crosslinked.
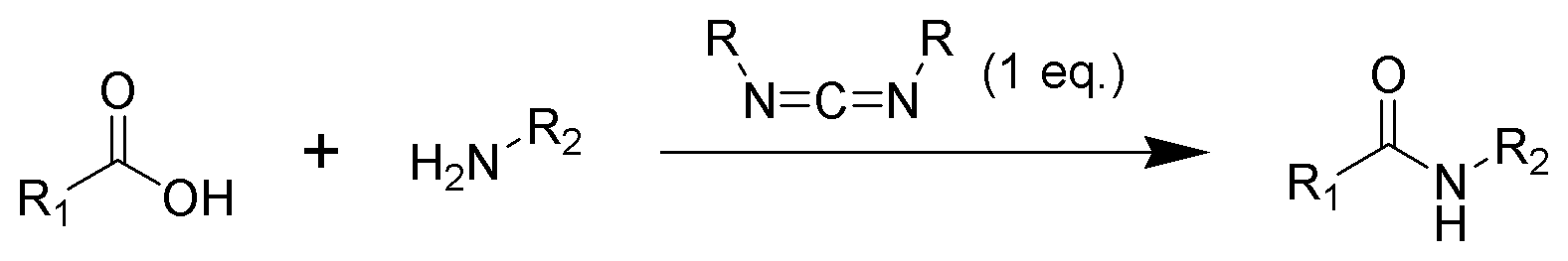
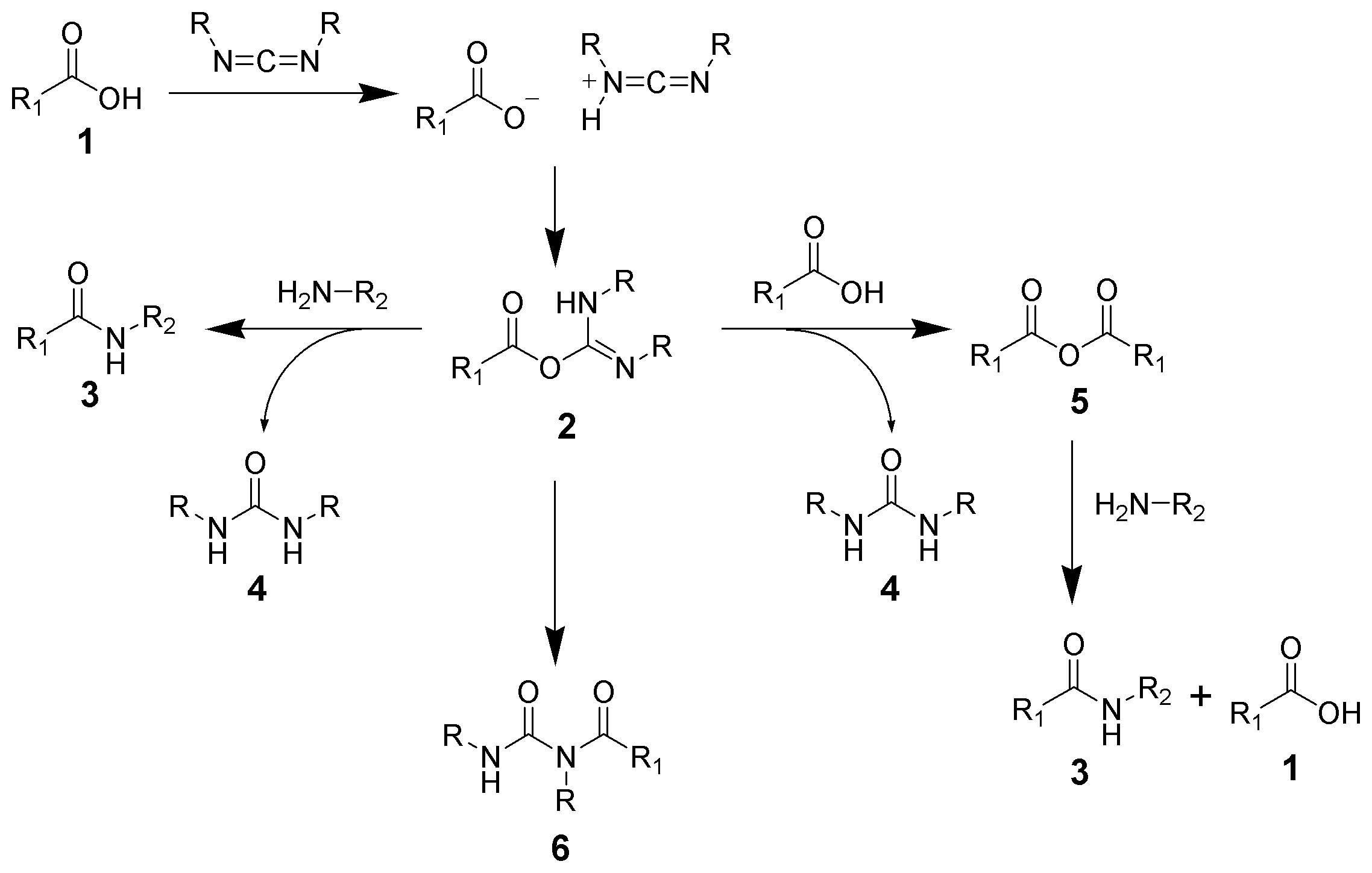
Amide formation pathway
The formation of an amide using a carbodiimide is a common reaction, but carries the risk of several side reactions. The acid 1 will react with the carbodiimide to produce the key intermediate: the O-acylisourea 2, which can be viewed as acarboxylic ester
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
with an activated leaving group. The O-acylisourea will react with amines to give the desired amide 3 and urea 4.
The possible reactions of the O-acylisourea 2 produce both desired and undesired products. The O-acylisourea 2 can react with an additional carboxylic acid 1 to give an acid anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of wa ...
5, which can react further to give the amide 3. The main undesired reaction pathway involves the rearrangement of the O-acylisourea 2 to the stable ''N''- acylurea 6. The use of solvents with low dielectric constants such as dichloromethane or chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
can minimize this side reaction.

Examples
DCC
 DCC (acronym for ''N'',''N''-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for
DCC (acronym for ''N'',''N''-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for solid-phase synthesis
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with ...
of peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...
s. DCC has achieved popularity mainly because of its high-yielding amide coupling reactions and the fact that it is quite inexpensive.
However, DCC does have some serious drawbacks, and its use is often avoided for several reasons:
#The byproduct ''N'',''N''- dicyclohexylurea is mostly removed by filtration, but trace impurities can be difficult to remove. It is incompatible with traditional solid-phase peptide synthesis.
#DCC is a potent allergen
An allergen is an otherwise harmless substance that triggers an allergic reaction in sensitive individuals by stimulating an immune response.
In technical terms, an allergen is an antigen that is capable of stimulating a type-I hypersensitivi ...
, and repeated contact with skin increases the probability of sensitization to the compound. Clinical reports of individuals who cannot enter rooms where peptide coupling agents are used have been reported.
DIC
 In contrast to DCC, DIC ( ''N'',''N''-diisopropylcarbodiimide) is a liquid. Its hydrolysis product, N,N'-diisopropylurea, is soluble in organic solvents.
In contrast to DCC, DIC ( ''N'',''N''-diisopropylcarbodiimide) is a liquid. Its hydrolysis product, N,N'-diisopropylurea, is soluble in organic solvents.
EDC
EDC is a water-soluble carbodiimide reagent used for a wide range of purposes. Apart from uses similar to those of DCC and DIC, it is also used for various biochemical purposes as a crosslinker or chemical probe.CMCT or CMC
1-cyclohexyl-(2-morpholinoethyl)carbodiimide metho-''p''-toluene sulfonate is a carbodiimide developed for the chemical probing of RNA structure in biochemistry.See also
* Sulfur diimide - the sulfur analogueReferences
{{Molecules detected in outer space Organic chemistry Functional groups