Noble Gas Compounds on:
[Wikipedia]
[Google]
[Amazon]
In
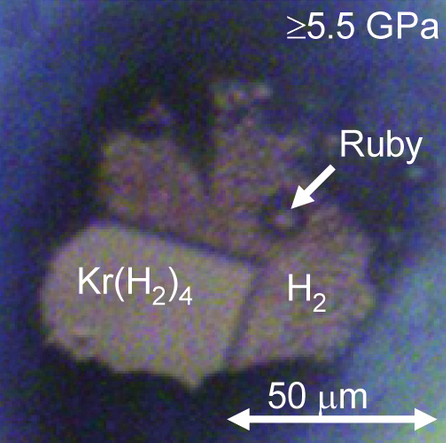
 Prior to 1962, the only isolated compounds of noble gases were
Prior to 1962, the only isolated compounds of noble gases were
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, noble gas compounds are chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s that include an element
Element or elements may refer to:
Science
* Chemical element, a pure substance of one type of atom
* Heating element, a device that generates heat by electrical resistance
* Orbital elements, parameters required to identify a specific orbit of o ...
from the noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es, group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
8 or 18 of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. Although the noble gases are generally unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
elements, many such compounds have been observed, particularly involving the element xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
.
From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
( ionisation energy 14.0 eV), xenon (12.1 eV), and radon
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only Rn has a sufficiently long half-life (3.825 days) for it to b ...
(10.7 eV) on one side, and the very unreactive argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
(15.8 eV), neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
(21.6 eV), and helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
(24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
, whereas He, Ne, Ar have been observed to form true chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s using spectroscopic
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectrosc ...
techniques, but only when frozen into a noble gas matrix at temperatures of or lower, in supersonic jets of noble gas, or under extremely high pressures with metals.
The heavier noble gases have more electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
s than the lighter ones. Hence, the outermost electrons are subject to a shielding effect
In chemistry, the shielding effect sometimes referred to as atomic shielding or
electron shielding describes the attraction between an electron and the nucleus in any atom with more than one electron. The shielding effect can be defined as a r ...
from the inner electrons that makes them more easily ionized
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
, since they are less strongly attracted to the positively-charged nucleus
Nucleus (: nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucleu ...
. This results in an ionization energy low enough to form stable compounds with the most electronegative elements, fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and even with less electronegative elements such as nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
under certain circumstances.
History and background
When the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed that they were all ''inert gases'' (as they were then known) which could not form compounds. With the development of atomic theory in the early twentieth century, their inertness was ascribed to a fullvalence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s which render them very chemically stable and nonreactive. All noble gases have full ''s'' and ''p'' outer electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
s (except helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, which has no ''p'' sublevel), and so do not form chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s easily. Their high ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
and almost zero electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
This differs by si ...
explain their non-reactivity.
In 1933, Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
predicted that the heavier noble gases would be able to form compounds with fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. Specifically, he predicted the existence of krypton hexafluoride () and xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All of them are exergonic and stable at normal temperatur ...
(), speculated that might exist as an unstable compound, and suggested that xenic acid would form perxenate salts. These predictions proved quite accurate, although subsequent predictions for indicated that it would be not only thermodynamically unstable, but kinetically unstable. As of 2022, has not been made, although the octafluoroxenate(VI) anion ( ) has been observed.
By 1960, no compound with a covalently bound noble gas atom had yet been synthesized. The first published report, in June 1962, of a noble gas compound was by Neil Bartlett, who noticed that the highly oxidising compound platinum hexafluoride
Platinum hexafluoride is the chemical compound with the formula Pt F6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation sta ...
ionised to . As the ionisation energy of to (1165 kJ mol−1) is nearly equal to the ionisation energy of Xe to (1170 kJ mol−1), he tried the reaction of Xe with . This yielded a crystalline product, xenon hexafluoroplatinate
Xenon hexafluoroplatinate is the product of the reaction of platinum hexafluoride with xenon, in an experiment that proved the chemical reactivity of the noble gases. This experiment was performed by Neil Bartlett at the University of British Co ...
, whose formula was proposed to be .
It was later shown that the compound is actually more complex, containing both and . Nonetheless, this was the first real compound of any noble gas.
The first binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two values (0 and 1) for each digit
* Binary function, a function that takes two arguments
* Binary operation, a mathematical op ...
noble gas compounds were reported later in 1962. Bartlett synthesized xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
: Xe + 2 →
This reaction is exothermic, rele ...
() by subjecting a mixture of xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and fluorine to high temperature. Rudolf Hoppe, among other groups, synthesized xenon difluoride
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as th ...
() by the reaction of the elements.
Following the first successful synthesis of xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
compounds, synthesis of krypton difluoride
Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances ...
() was reported in 1963.
True noble gas compounds
In this section, the non-radioactive noble gases are considered in decreasing order ofatomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
, which generally reflects the priority of their discovery, and the breadth of available information for these compounds. The radioactive elements radon and oganesson are harder to study and are considered at the end of the section.
Xenon compounds
After the initial 1962 studies on and , xenon compounds that have been synthesized include other fluorides ( ), oxyfluorides ( , , , , ) and oxides ( , and ). Xenon fluorides react with several other fluorides to form fluoroxenates, such as sodium octafluoroxenate(VI) (), and fluoroxenonium salts, such as trifluoroxenonium hexafluoroantimonate (). In terms of other halide reactivity, short-livedexcimer
An excimer (originally short for excited dimer) is a short-lived polyatomic molecule formed from two species that do not form a stable molecule in the ground state. In this case, formation of molecules is possible only if such atom is in an elec ...
s of noble gas halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
such as or XeCl are prepared in situ, and are used in the function of excimer laser
An excimer laser, sometimes more correctly called an exciplex laser, is a form of ultraviolet laser which is commonly used in the production of microelectronic devices, semiconductor based integrated circuits or "chips", eye surgery, and micro ...
s.
Recently, xenon has been shown to produce a wide variety of compounds of the type where ''n'' is 1, 2 or 3 and X is any electronegative group, such as , , , , , , etc.; the range of compounds is impressive, similar to that seen with the neighbouring element iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
, running into the thousands and involving bonds between xenon and oxygen, nitrogen, carbon, boron and even gold, as well as perxenic acid, several halides, and complex ions.
The compound contains a Xe–Xe bond, which is the longest element-element bond known (308.71 pm = 3.0871 Å). Short-lived excimer
An excimer (originally short for excited dimer) is a short-lived polyatomic molecule formed from two species that do not form a stable molecule in the ground state. In this case, formation of molecules is possible only if such atom is in an elec ...
s of are reported to exist as a part of the function of excimer laser
An excimer laser, sometimes more correctly called an exciplex laser, is a form of ultraviolet laser which is commonly used in the production of microelectronic devices, semiconductor based integrated circuits or "chips", eye surgery, and micro ...
s.
Krypton compounds
Krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
gas reacts with fluorine gas under extreme forcing conditions, forming according to the following equation:
:
reacts with strong Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s to form salts of the and cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s. The preparation of reported by Grosse in 1963, using the Claasen method, was subsequently shown to be a mistaken identification.
Krypton compounds with other than Kr–F bonds (compounds with atoms other than fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
) have also been described. reacts with to produce the unstable compound, , with a krypton-oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
bond. A krypton-nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
bond is found in the cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, produced by the reaction of with below −50 °C.
Argon compounds
The discovery of HArF was announced in 2000. The compound can exist in low temperatureargon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
matrices
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the ...
for experimental studies, and it has also been studied computationally. Argon hydride ion was obtained in the 1970s.
This molecular ion has also been identified in the Crab nebula
The Crab Nebula (catalogue designations M1, NGC 1952, Taurus A) is a supernova remnant and pulsar wind nebula in the constellation of Taurus (constellation), Taurus. The common name comes from a drawing that somewhat resembled a crab with arm ...
, based on the frequency of its light emissions.
There is a possibility that a solid salt of could be prepared with or anions.
Neon and helium compounds
The ions, , , , and are known from optical and mass spectrometric studies. Neon also forms an unstable hydrate. There is some empirical and theoretical evidence for a few metastablehelium compounds
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 ...
which may exist at very low temperatures or extreme pressures. The stable cation was reported in 1925, but was not considered a true compound since it is not neutral and cannot be isolated. In 2016 scientists created the helium compound disodium helide
Disodium helide (Na2He) is a compound of helium and sodium that is stable at high pressures above . It was first predicted using the USPEX crystal structure prediction algorithm and then synthesised in 2016.
Synthesis
Na2He was predicted to be t ...
() which was the first helium compound discovered.
Radon and oganesson compounds
Radon
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only Rn has a sufficiently long half-life (3.825 days) for it to b ...
is not chemically inert, but its short half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
(3.8 days for 222Rn) and the high energy of its radioactivity make it difficult to investigate its only fluoride (), its reported oxide (), and their reaction products.
All known oganesson
Oganesson is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint ...
isotopes have even shorter half-lives in the millisecond range and no compounds are known yet, although some have been predicted theoretically. It is expected to be even more reactive than radon, more like a normal element than a noble gas in its chemistry.
Reports prior to xenon hexafluoroplatinate and xenon tetrafluoride
Clathrates

 Prior to 1962, the only isolated compounds of noble gases were
Prior to 1962, the only isolated compounds of noble gases were clathrates
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin (), meaning 'with bars, latticed'. Most clathrate compounds are polymeric and completely envelop the ...
(including clathrate hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
s); other compounds such as coordination compounds
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
were observed only by spectroscopic means. Clathrates (also known as cage compounds) are compounds of noble gases in which they are trapped within cavities of crystal lattices of certain organic and inorganic substances. Ar, Kr, Xe and Ne can form clathrates with crystalline hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para' ...
. Kr and Xe can appear as guests in crystals of melanophlogite
Melanophlogite (MEP) is a rare silicate mineral and a polymorph of silica (SiO2). It has a zeolite-like porous structure which results in relatively low and not well-defined values of its density and refractive index. Melanophlogite often overgrow ...
.
Helium-nitrogen () crystals have been grown at room temperature at pressures ca. 10 GPa in a diamond anvil cell
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It permits the compression of a small (sub- millimeter-sized) piece of material to extreme pressures, typically up to around 1 ...
. Solid argon-hydrogen clathrate () has the same crystal structure as the Laves phase
Laves phases are intermetallic phase (matter), phases that have composition AB2 and are named for Fritz Laves who first described them. The phases are classified on the basis of geometry alone. While the problem of Close-packing of equal spheres ...
. It forms at pressures between 4.3 and 220 GPa, though Raman measurements suggest that the molecules in dissociate above 175 GPa. A similar solid forms at pressures above 5 GPa. It has a face-centered cubic structure where krypton octahedra are surrounded by randomly oriented hydrogen molecules. Meanwhile, in solid xenon atoms form dimers inside solid hydrogen
Solid hydrogen is the solid state of the element hydrogen. At standard pressure, this is achieved by decreasing the temperature below hydrogen's melting point of . It was collected for the first time by James Dewar in 1899 and published with the ...
.
Coordination compounds
Coordination compounds such as have been postulated to exist at low temperatures, but have never been confirmed.Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
is known to function as a metal ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
. In addition to the charged uXe4sup>2+, xenon, krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
, and argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
all reversibly bind to gaseous M(CO)5, where M=Cr, Mo, or W. ''P''-block metals also bind noble gases: XeBeO has been observed spectroscopically and both XeBeS and FXeBO are predicted stable.
Also, compounds such as and were reported to have been formed by electron bombardment, but recent research has shown that these are probably the result of He being adsorb
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
ed on the surface of the metal; therefore, these compounds cannot truly be considered chemical compounds.
Hydrates
Hydrates are formed by compressing noble gases in water, where it is believed that the water molecule, a strong dipole, induces a weak dipole in the noble gas atoms, resulting in dipole-dipole interaction. Heavier atoms are more influenced than smaller ones, hence was reported to have been the most stable hydrate; it has a melting point of 24 °C. The deuterated version of this hydrate has also been produced.Fullerene adducts
Noble gases can also formendohedral fullerene
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in t ...
compounds where the noble gas atom is trapped inside a fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
molecule. In 1993, it was discovered that when is exposed to a pressure of around 3 bar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
** Chocolate bar
* Protein bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a laye ...
of He or Ne, the complexes and are formed. Under these conditions, only about one out of every 650,000 cages was doped with a helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
atom; with higher pressures (3000 bar), it is possible to achieve a yield of up to 0.1%. Endohedral complexes with argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
, krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
and xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
have also been obtained, as well as numerous adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
s of .
Applications
Most applications of noble gas compounds are either as oxidising agents or as a means to store noble gases in a dense form. Xenic acid is a valuable oxidising agent because it has no potential for introducing impurities—xenon is simply liberated as a gas—and so is rivalled only byozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
in this regard. The perxenates are even more powerful oxidizing agents. Xenon-based oxidants have also been used for synthesizing carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s stable at room temperature, in solution.
Stable salts of xenon containing very high proportions of fluorine by weight (such as tetrafluoroammonium
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced ...
heptafluoroxenate(VI), , and the related tetrafluoroammonium octafluoroxenate(VI) ), have been developed as highly energetic oxidisers for use as propellants in rocketry.
Xenon fluorides are good fluorinating agents.
Clathrates have been used for separation of He and Ne from Ar, Kr, and Xe, and also for the transportation of Ar, Kr, and Xe. (For instance, radioactive isotopes of krypton and xenon are difficult to store and dispose, and compounds of these elements may be more easily handled than the gaseous forms.) In addition, clathrates of radioisotopes may provide suitable formulations for experiments requiring sources of particular types of radiation; hence. 85Kr clathrate provides a safe source of beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and Π...
s, while 133Xe clathrate provides a useful source of gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s.
References
Resources
* {{Chemical compounds by element N