Nickel Boride Catalyst on:
[Wikipedia]
[Google]
[Amazon]
Nickel boride is the common name of materials composed chiefly of the elements

 Ni2B can also be used to cleave
Ni2B can also be used to cleave
 For aryl bromides, the modified system in dimethylformamide is used for clean debromination. Reductive cleavage of iodides occurs with retention of configuration.
For aryl bromides, the modified system in dimethylformamide is used for clean debromination. Reductive cleavage of iodides occurs with retention of configuration.
Raymond Paul, Paul Buisson, and Nicole Joseph (1952): "Catalytic activity of nickel borides". ''Industrial and Engineering Chemistry'', volume 44, issue 5, pages 1006-1010.
Hermann Irving Schlesinger, Herbert C. Brown, A. E. Finholt, James R. Gilbreath, Henry R. Hoekstra, and Earl K. Hyde (1953): "Sodium borohydride its hydrolysis and its use as a reducing agent and in the generation of hydrogen". ''Journal of the American Chemical Society'', volume 75, issue 1,pages 215-219.
Charles A. Brown and Herbert C. Brown (1963): "The reaction of sodium borohydride with nickel acetate in aqueous solution—a convenient synthesis of a nickel hydrogenation catalyst of low isomerization tendency". ''Journal of the American Chemical Society'' (Communications to the Editor), volume 85, issue 7, pages 1003-1005.
Herbert C. Brown and Charles A. Brown (1963): "The reaction of sodium borohydride with nickel acetate in ethanol solution: a highly selective nickel hydrogenation catalyst". ''Journal of the American Chemical Society'' (Communications to the Editor), volume 85, issue 7, pages 1005-1006.
L. J. E. Hofer, J. F. Shultz, R. D. Panson, and R. B. Anderson (1964): "The nature of the nickel boride formed by the action of sodium borohydride on nickel salts". ''Inorganic Chemistry'', volume 3, issue 12, pages 1783–1785.
Charles Allan Brown (1970): "Catalytic hydrogenation. V. Reaction of sodium borohydride with aqueous nickel salts. P-1 nickel boride, a convenient, highly active nickel hydrogenation catalyst". ''
{{Borides
Borides
Nickel compounds
Hydrogenation catalysts
nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
and boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
that are widely used as catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. Their approximate chemical composition
A chemical composition specifies the identity, arrangement, and ratio of the chemical elements making up a compound by way of chemical and atomic bonds.
Chemical formulas can be used to describe the relative amounts of elements present in a com ...
is Ni2.5B, and they are often incorrectly denoted "" in organic chemistry publications.
Nickel boride catalysts are typically prepared by reacting a salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of nickel with sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
. The composition and properties vary depending on the specific preparation method. The two most common forms, described and evaluated in detail by Herbert C. Brown and Charles Allan Brown in 1963, are known as P−1 nickel and P−2 nickel.
These catalysts are usually obtained as black granules (P−1) or colloidal suspensions (P−2). They are air-stable, non-magnetic
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, m ...
and non-pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
, but slowly react with water to form nickel hydroxide
Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is a lime-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide ...
. They are insoluble in all solvents, but react with concentrated mineral acids
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Cha ...
. They are claimed to be more effective hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
catalysts than Raney nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable s ...
.
History
These catalysts originate duringWorld War II
World War II or the Second World War (1 September 1939 – 2 September 1945) was a World war, global conflict between two coalitions: the Allies of World War II, Allies and the Axis powers. World War II by country, Nearly all of the wo ...
with the work of a research group led by Hermann I. Schlesinger, discoverer of borohydrides. They noted that reaction of with salts of certain transition metals yielded black precipitates, and that the cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
product catalyzed the decomposition of borohydride. However, their research was focused on war-related applications, and the black precipitate was not investigated further.
In 1951, Raymond Paul
Raymond Rudolf Valentine Paul (21 November 1928 – 23 December 2013) was a British fencer.
Fencing career
He competed at the 1952 and 1956 Summer Olympics with the best individual result of eighth place in the foil in 1956. He won four na ...
and others investigated the reaction of with nickel chloride, sulfate, and acetate in various solvents and measured their performance as hydrogenation catalysts.
In 1963, H. C. Brown and Charles A. Brown reported the synthesis and performance of two similar catalysts, which they denoted by "P-1" (the same as Paul's) and "P-2", obtained by reacting sodium borohydride with nickel acetate in water and ethanol, respectively.
Preparation
In contrast with otherboride
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some b ...
s, which require high temperatures, preparation of these nickel boride catalysts can be carried out at ambient temperature, without special equipment. Indeed, they are usually generated ''in situ''.
The P−1 catalyst can be generated by reacting a nickel(II) salt, such as sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
, or acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
, and sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
in alkaline aqueous solutions. The product precipitates as a fine, black granular powder. The chemistry is very similar to that of electroless nickel-boron plating, and yields hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas and the corresponding sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
salt as byproducts. The borohydride must be added gradually to the nickel salt solution, not the other way around, because the product catalyzes the hydrolysis of borohydride to hydrogen and hypoborate . The catalytic activity of P-1 is enhanced by adding small amount of salts of other metals (but not cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
) to the nickel salt during preparation. Benzene however reduces its activity somewhat.
The P−2 form is prepared similarly from nickel(II) acetate and sodium borohydride in ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
. An inert atmosphere was found necessary for maximum catalytic activity. The result was an almost colloidal suspension of the black catalyst. Another method uses nickel chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemi ...
NiCl2 instead of acetate.
Structure and composition
The P-1 and P-2 "nickel boride" catalyst have been suggested to be amorphous compounds, composed of nickel bonded to individual boron centres. However, that structure was later found to be incorrect. AnX-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
analysis of P-1 by L. Hofer and others in 1964 indicated that the nickel and boron contents were in 2.5:1 ratio, but the solid contained 11% of strongly bound water and other compounds. was amorphous when freshly prepared (with crystalline nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s about 1.5 nm across), but even heating at 90 °C caused the formation of some crystalline nickel. Heating at 250 °C caused it to separate into two phases: metallic nickel, and crystalline trinickel boride with the cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, b ...
structure, stable at least up to 750 C. No trace of the true dinickel boride was seen. The authors concluded that P-1 was an intimate mixture of metallic nickel and some amorphous boron-containing compound.
The true structure of these "nickel borides" was elucidated only in 2007. They consist of small grains of crystalline nickel boride embedded in an amorphous nickel matrix.
The two forms P−1 and P−2 differ in terms of amount of their contamination by NaBO2 adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
on the surface. P−1 Ni2B has an oxide to boride ratio of 1:4, whereas that of P−2 Ni2B is 10:1. Their properties differ in terms of catalytic efficiency and substrate specificity.
Applications
Ni2B is an efficientcatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
and reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
. It is used as a heterogeneous hydrogenation catalyst.
Catalytic hydrogenation
The catalytic activity of P−1 is insensitive tosteric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
of side chains on the substrate and thus more active, and seldom affects protecting groups. In contrast, P−2 is very sensitive to steric factors. For these reasons, P−1 is usually used for the complete reduction of unsaturated hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may b ...
under mild conditions, while P−2 is useful in partial reductions such as converting alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s to alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s in high yields:
The H2/Ni2B system will not hydrogenolyse ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s, alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s, aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s, amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s as it reduces alkenes in preference, even under forcing conditions. It leaves epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s unaffected, but affects cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
s occasionally. Most ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s are stable to Ni2B, except for benzylic, allylic and propargylic esters which are cleaved by hydrogenolysis:

Desulfurization
The NiCl2/NaBH4 system desulfurizesthioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond charact ...
s, thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s, thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
s, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s and sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s. Organic sulfides, disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
s, thiols, and sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s are reduced by NiCl2/NaBH4 to hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s. Illustrated is the reduction of phenothiazine
Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history.
...
to diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidiz ...
:
 Ni2B can also be used to cleave
Ni2B can also be used to cleave thioacetal
In organosulfur chemistry, thioacetals are the sulfur (''thio-'') analog (chemistry), analogues of acetals (). There are two classes: the less-common monothioacetals, with the formula , and the dithioacetals, with the formula (symmetric dithio ...
s. Since Ni2B is non-pyrophoric, stable in air, and give high yields in many cases, it is proposed as a safer alternative to Raney Nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable s ...
for removal of cyclic thioacetals. Desulfurization catalyzed by Ni2B proved to occur with retention of configuration by isotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' ...
.
Reduction of nitrogenous groups
The NiCl2/NaBH4 system reduces aliphaticnitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nit ...
s, nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
s and oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
s completely to amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s. For aryl amines, nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced ...
s are converted to aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
s, and azoxy
In chemistry, azoxy compounds are a group of organic compounds sharing a common functional group with the general structure . They are considered Amine oxide, N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles and 1,3-dipolar cycloadditio ...
benzenes to azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide c ...
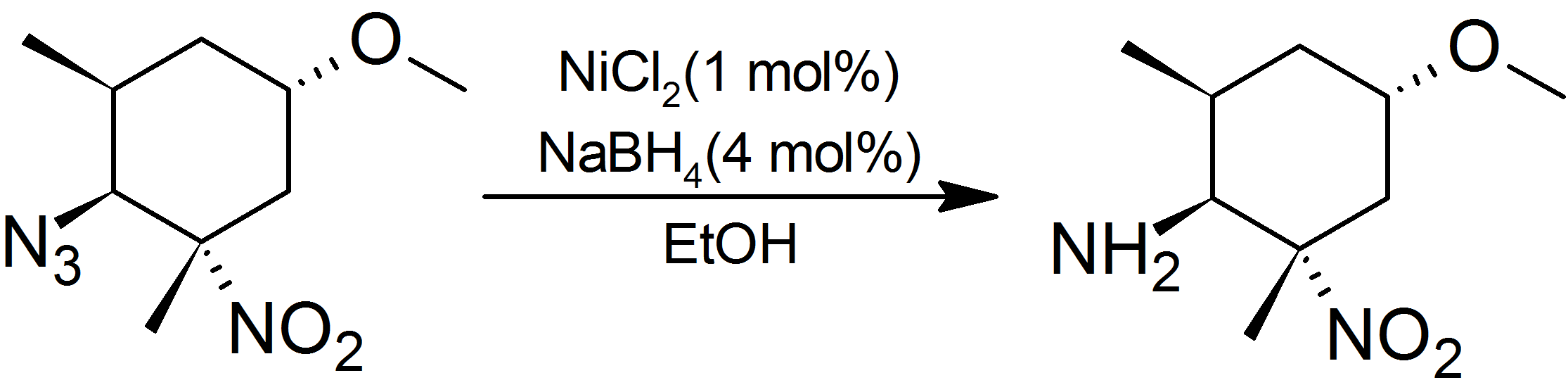
s. Azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s are cleanly reduced to amines in preference to sterically hindered
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
aliphatic nitro groups:

Dehalogenation
Most organicfluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typi ...
and chlorides
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
are unaffected by Ni2B, bromides
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardan ...
show variable reactivity, and iodides
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency a ...
are often completely reduced to hydrocarbons. With Ni2B in dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
, α-bromoketones are reduced to the parent ketones. Vicinal bromides are dehalogenated to alkenes:
 For aryl bromides, the modified system in dimethylformamide is used for clean debromination. Reductive cleavage of iodides occurs with retention of configuration.
For aryl bromides, the modified system in dimethylformamide is used for clean debromination. Reductive cleavage of iodides occurs with retention of configuration.
Safety
Nickel compounds are possible carcinogens and contact with skin should be avoided. Particular care should be taken whenever is used in dimethylformamide assodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
may spontaneously ignite in dimethylformamide.
See also
* Cobalt boride *Urushibara nickel
Urushibara nickel is a nickel-based hydrogenation catalyst. It is a heterogeneous catalyst, comparable to Raney nickel. Urushibara nickel is however not pyrophoric. For most hydrogenations, it performs comparably to W-7 grade Raney nickel.
Prepar ...
References
The Journal of Organic Chemistry
''The Journal of Organic Chemistry'', colloquially known as ''JOC'', is a peer-reviewed scientific journal for original contributions of fundamental research in all branches of theory and practice in organic and bioorganic chemistry. It is publ ...
'', volume 35, issue 6, pages 1900–1904.
Charles Allan Brown and Vijay K. Ahuja (1973): "Catalytic hydrogenation. VI. Reaction of sodium borohydride with nickel salts in ethanol solution. P-2 Nickel, a highly convenient, new, selective hydrogenation catalyst with great sensitivity to substrate structure". ''Journal of Organic Chemistry'', volume 38, issue 12, pages 2226–2230.
Chemicals & Reagents, 2008-2010