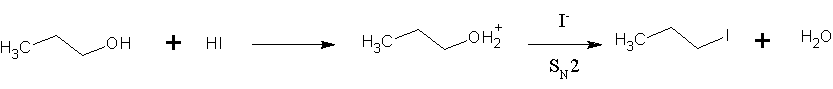|
Diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized impurities.P. F. Vogt, J. J. Gerulis, "Amines, Aromatic" in Ullmann’s Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. Diphenylamine dissolves well in many common organic solvents, and is moderately soluble in water. It is used mainly for its antioxidant properties. Diphenylamine is widely used as an industrial antioxidant, dye mordant and reagent and is also employed in agriculture as a fungicide and antihelmintic. Preparation and reactivity Diphenylamine is manufactured by the thermal deamination of aniline over oxide catalysts: : 2 C6H5NH2 → (C6H5)2NH + NH3 It is a weak base, with a ''K''b of 10−14. With strong acids, it forms salts. For example, treatment with sulfuric acid gives the bisulfate C6H5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smokeless Powder
Finnish smokeless powderSmokeless powder is a type of propellant used in firearms and artillery that produces less smoke and less fouling when fired compared to gunpowder ("black powder"). The combustion products are mainly gaseous, compared to around 55% solid products (mostly potassium carbonate, potassium sulfate, and potassium sulfide) for black powder. In addition, smokeless powder does not leave the thick, heavy fouling of hygroscopic material associated with black powder that causes rusting of the barrel. Despite its name, smokeless powder is not completely free of smoke; while there may be little noticeable smoke from small-arms ammunition, smoke from artillery fire can be substantial. Originally invented in 1884 by Paul Vieille, the most common formulations are based on nitrocellulose, but the term was also used to describe various picrate mixtures with nitrate, chlorate, or dichromate oxidizers during the late 19th century, before the advantages of nitrocellu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate Test
A Nitrate Test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium. The nitrate anion is an oxidizer, and many tests for the nitrate anion are based on this property. However, other oxidants present in the analyte may interfere and give erroneous results. Nitrate can also be detected by first reducing it to the more reactive nitrite ion and using one of many nitrite tests. Brown ring test A common nitrate test, known as the brown ring testEgon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier can be performed by adding iron(II) sulfate to a solution of a nitrate, then slowly adding concentrated sulfuric acid such that the acid forms a layer bel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo vario ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenothiazine
Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history. The derivatives chlorpromazine and promethazine revolutionized the fields of psychiatry and allergy treatment, respectively. An earlier derivative, methylene blue, was one of the first antimalarial drugs, and derivatives are under investigation as possible anti-infective drugs. Phenothiazine is a prototypical pharmaceutical lead structure in medicinal chemistry. Uses Phenothiazine itself is only of theoretical interest, but its derivatives revolutionized psychiatry, other fields of medicine, and pest management. Other derivatives have been studied for possible use in advanced batteries and fuel cells. Phenothiazine-derived drugs In 1876, methylene blue, a derivative of phenothiazine, was synthesized by Heinrich Caro at BASF. The st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylamine
Triphenylamine is an organic compound with formula (C6H5)3N. In contrast to most amines, triphenylamine is nonbasic. At room temperature it appears as a colorless crystalline solid, with monoclinic structure. It is well miscible in diethyl ether and benzene, but it is practically insoluble in water, and partially in ethanol. Its derivatives have useful properties in electrical conductivity and electroluminescence, and they are used in OLEDs as hole-transporters. Triphenylamine can be prepared by arylation of diphenylamine. Physical properties Triphenylamine has three aromatic groups directly linked to the central nitrogen atom. Each aromatic group acts as an electron attractor, directing the electron cloud of the lone pair of nitrogen towards it. With the delocalization of the nitrogen lone pair,T. Zhang, I. E. Brumboiu, C. Grazioli, A. Guarnaccio, M. Coreno, M. de Simone, A. Santagata, H. Rensmo, B. Brena, V. Lanzilotto, and C. Puglia "Lone-Pair Delocalization Effects within ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substitue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Indicator
A redox indicator (also called an oxidation-reduction indicator) is an indicator which undergoes a definite color change at a specific electrode potential. The requirement for fast and reversible color change means that the oxidation-reduction equilibrium for an indicator redox system needs to be established very quickly. Therefore, only a few classes of organic redox systems can be used for indicator purposes. There are two common classes of redox indicators: * metal complexes of phenanthroline and bipyridine. In these systems, the metal changes oxidation state. * organic redox systems such as methylene blue. In these systems, a proton participant in the redox reaction. Therefore, sometimes redox indicators are also divided into two general groups: independent or dependent on pH. The most common redox indicator are organic compounds. Redox Indicator example: The molecule 2,2'- Bipyridine is a redox Indicator. In solution, it changes from light blue to red at an electrode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substitue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is not pure hydrogen iodide, but a mixture containing it. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylation
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodobenzene
Iodobenzene is an organoiodine compound consisting of a benzene ring substituted with one iodine atom. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish. Preparation Iodobenzene is commercially available, but it can be prepared in the laboratory from aniline via the Diazotization Reaction. In the first step, the amine functional group is diazotized with hydrochloric acid and sodium nitrite. Potassium iodide is added to the resultant phenyldiazonium chloride, causing nitrogen gas to evolve. The product is separated by steam distillation. : Alternatively, it can be produced by refluxing iodine and nitric acid with benzene. Reactions Since the C–I bond is weaker than C–Br or C–Cl, iodobenzene is more reactive than bromobenzene or chlorobenzene. Iodobenzene reacts readily with magnesium to form the Grignard reagent, phenylmagnesium iodide. Phenylmagnesium iodide, like the bromide analog, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |