Neptunium(IV) Peroxide on:
[Wikipedia]
[Google]
[Amazon]
Neptunium is a
Rn">Radon.html" ;"title="/nowiki>Radon">Rn/nowiki> 5f4 6d1 7s2. This differs from the configuration expected by the Aufbau principle in that one electron is in the 6d Electron shell#Subshells, subshell instead of being as expected in the 5f subshell. This is because of the similarity of the electron energies of the 5f, 6d, and 7s subshells. In forming compounds and ions, all the valence electrons may be lost, leaving behind an inert core of inner electrons with the electron configuration of the
 When the first
When the first
 As research on nuclear fission progressed in early 1939,
As research on nuclear fission progressed in early 1939, + -> -> beta^-23\ \ce] -> beta^-2.355\ \ce] ''(The times are half-life, half-lives.)''
This proved that the unknown radioactive source originated from the decay of uranium and, coupled with the previous observation that the source was different chemically from all known elements, proved beyond all doubt that a new element had been discovered. McMillan and Abelson published their results in a paper entitled ''Radioactive Element 93'' in the ''
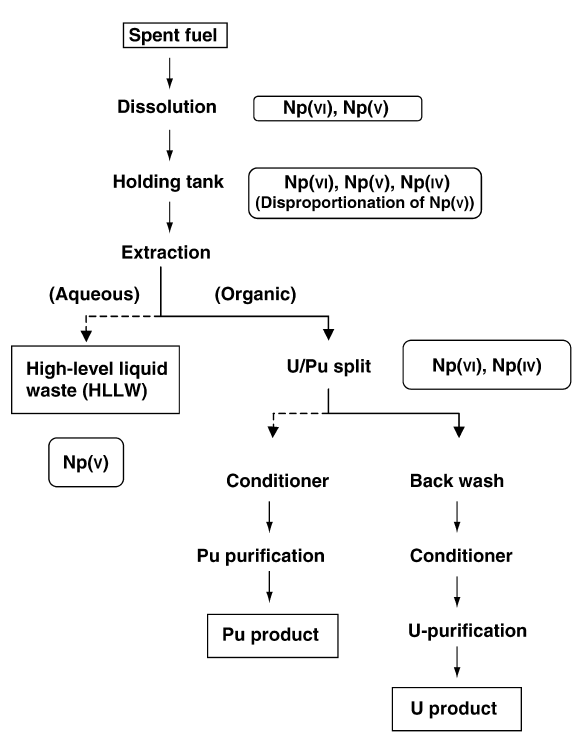
 When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of
When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of
 A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
^_Np + ^_n -> ^_Np -> beta^-2.117 \ \ce] ^_Pu
238Pu also exists in sizable quantities in
accessed Sept 23, 2006. It is not believed that an actual weapon has ever been constructed using neptunium. As of 2009, the world production of neptunium-237 by commercial power reactors was over 1000 critical masses a year, but to extract the isotope from irradiated fuel elements would be a major industrial undertaking. In September 2002, researchers at the Los Alamos National Laboratory briefly produced the first known nuclear
Neptunium
at ''The Periodic Table of Videos'' (University of Nottingham)
Lab builds world's first neptunium sphere
, U.S. Department of Energy Research News
NLM Hazardous Substances Databank – Neptunium, Radioactive
Neptunium: Human Health Fact Sheet
{{Authority control Neptunium, Chemical elements Actinides Synthetic elements Nuclear materials Pyrophoric materials
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Np and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
93. A radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
metal, neptunium is the first transuranic element
The transuranium (or transuranic) elements are the chemical elements with atomic number greater than 92, which is the atomic number of uranium. All of them are radioactively unstable and decay into other elements. Except for neptunium and pluton ...
. It is named after Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
, the planet beyond Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
in the Solar System, which uranium is named after. A neptunium atom has 93 proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and 93 electrons, of which seven are valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s. Neptunium metal is silvery and tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
es when exposed to air. The element occurs in three allotropic
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
forms and it normally exhibits five oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s, ranging from +3 to +7. Like all actinides, it is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, poisonous
A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figurati ...
, pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
, and capable of accumulating in bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s, which makes the handling of neptunium dangerous.
Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan
Edwin Mattison McMillan (September 18, 1907 – September 7, 1991) was an American physicist credited with being the first to produce a transuranium element, neptunium. For this, he shared the 1951 Nobel Prize in Chemistry with Glenn Seaborg.
...
and Philip H. Abelson at the Berkeley Radiation Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a federally funded research and development center in the hills of Berkeley, California, United States. Established in 1931 by the University of California (UC), the laboratory is spo ...
in 1940. Since then, most neptunium has been and still is produced by neutron irradiation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitti ...
of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
reactors. While neptunium itself has no commercial uses at present, it is used as a precursor for the formation of plutonium-238
Plutonium-238 ( or Pu-238) is a radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suitable for usage ...
, which is in turn used in radioisotope thermal generator
A radioisotope thermoelectric generator (RTG, RITEG), or radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the Decay heat, heat released by the decay of a suitable radioactive material i ...
s to provide electricity for spacecraft
A spacecraft is a vehicle that is designed spaceflight, to fly and operate in outer space. Spacecraft are used for a variety of purposes, including Telecommunications, communications, Earth observation satellite, Earth observation, Weather s ...
. Neptunium has also been used in detector
A sensor is often defined as a device that receives and responds to a signal or stimulus. The stimulus is the quantity, property, or condition that is sensed and converted into electrical signal.
In the broadest definition, a sensor is a devi ...
s of high-energy neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s.
The longest-lived isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of neptunium, neptunium-237, is a by-product of nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
production. This isotope, and the isotope neptunium-239, are also found in trace amounts in uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
ores due to neutron capture reactions and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
.
__TOC__
Characteristics
Physical
Neptunium is ahard
Hard means something that is difficult to do. It may also refer to:
* Hardness, resistance of physical materials to deformation or fracture
* Hard water, water with high mineral content
Arts and entertainment
* Hard (TV series), ''Hard'' (TV ser ...
, silvery, ductile
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
, radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
actinide metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
. In the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, it is located to the right of the actinide uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, to the left of the actinide plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
and below the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
. Neptunium is a hard metal, having a bulk modulus of 118 GPa
Grading in education is the application of standardized measurements to evaluate different levels of student achievement in a course. Grades can be expressed as letters (usually A to F), as a range (for example, 1 to 6), percentages, or as num ...
, comparable to that of manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
. Neptunium metal is similar to uranium in terms of physical workability. When exposed to air at normal temperatures, it forms a thin oxide layer. This reaction proceeds more rapidly as the temperature increases. Neptunium melts at : this low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
, a property the metal shares with the neighboring element plutonium (which has melting point 639.4 °C), is due to the hybridization of the 5f and 6d orbitals and the formation of directional bonds in the metal. The boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of neptunium is not empirically known and the usually given value of 4174 °C is extrapolated from the vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
of the element. If accurate, this would give neptunium the largest liquid range of any element (3535 K passes between its melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s).
Neptunium is found in at least three allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
s. Some claims of a fourth allotrope have been made, but they are so far not proven.Yoshida et al., p. 718. This multiplicity of allotropes is common among the actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s. The crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
s of neptunium, protactinium
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which p ...
, uranium, and plutonium do not have clear analogs among the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s and are more similar to those of the 3d transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s.
α-neptunium takes on an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
structure, resembling a highly distorted body-centered cubic structure.Lemire, R. J. et al.,''Chemical Thermodynamics of Neptunium and Plutonium'', Elsevier, Amsterdam, 2001. Each neptunium atom is coordinated to four others and the Np–Np bond lengths are 260 pm.Yoshida et al., p. 719. It is the densest of all the actinides and the fifth-densest of all naturally occurring elements, behind only rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
, and osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
.Theodore Gray. ''The Elements''. Page 215. α-neptunium has semimetal
A semimetal is a material with a small energy overlap between the bottom of the Electrical conduction, conduction Electronic band structure, band and the top of the valence band, but they do not overlap in momentum space. According to Band theory ...
lic properties, such as strong covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
ing and a high electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
, and its metallic physical properties are closer to those of the metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
s than the true metals. Some allotropes of the other actinides also exhibit similar behaviour, though to a lesser degree. The densities of different isotopes of neptunium in the alpha phase are expected to be observably different: α-235Np should have density 20.303 g/cm3; α-236Np, density 20.389 g/cm3; α-237Np, density 20.476 g/cm3.
β-neptunium takes on a distorted tetragonal close-packed structure. Four atoms of neptunium make up a unit cell, and the Np–Np bond lengths are 276 pm. γ-neptunium has a body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
structure and has Np–Np bond length of 297 pm. The γ form becomes less stable with increased pressure, though the melting point of neptunium also increases with pressure. The β-Np/γ-Np/liquid triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
occurs at 725 °C and 3200 MPa
MPA or mPa may refer to:
Academia
Academic degrees
* Master of Performing Arts
* Master of Professional Accountancy
* Master of Public Administration
* Master of Public Affairs
Schools
* Mesa Preparatory Academy
* Morgan Park Academy
* M ...
.
Alloys
Due to the presence of valence 5f electrons, neptunium and its alloys exhibit a very interesting magnetic behavior, like many other actinides. These can range from the itinerant band-like character characteristic of thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s to the local moment behavior typical of scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, and the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s. This stems from 5f-orbital hybridization with the orbitals of the metal ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s, and the fact that the 5f orbital is relativistically destabilized and extends outwards.Yoshida et al., pp. 719–20. For example, pure neptunium is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
, Np Al3 is ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ...
, Np Ge3 has no magnetic ordering, and Np Sn3 may be a heavy fermion material
In materials science, heavy fermion materials are a specific type of intermetallic compound, containing elements with 4f or 5f electrons in unfilled electron bands. Electrons are one type of fermion, and when they are found in such materials, the ...
. Investigations are underway regarding alloys of neptunium with uranium, americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
, plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, and iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, so as to recycle long-lived waste isotopes such as neptunium-237 into shorter-lived isotopes more useful as nuclear fuel.
One neptunium-based superconductor alloy has been discovered with formula Np Pd5Al2. This occurrence in neptunium compounds is somewhat surprising because they often exhibit strong magnetism, which usually destroys superconductivity. The alloy has a tetragonal structure with a superconductivity transition temperature of −268.3 °C (4.9 K).
Chemical
Neptunium has five ionicoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
s.
Atomic
A neptunium atom has 93 electrons, arranged in theconfiguration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
radon; more commonly, only some of the valence electrons will be lost. The electron configuration for the tripositive ion Np3+ is nnbsp;5f4, with the outermost 7s and 6d electrons lost first: this is exactly analogous to neptunium's lanthanide homolog promethium, and conforms to the trend set by the other actinides with their nnbsp;5f''n'' electron configurations in the tripositive state. The first ionization potential
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
of neptunium was measured to be at most in 1974, based on the assumption that the 7s electrons would ionize before 5f and 6d; more recent measurements have refined this to 6.2657 eV.
Isotopes
Twenty-four neptuniumradioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s have been characterized, with the most stable being 237Np with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 2.14 million years, 236Np with a half-life of 154,000 years, and 235Np with a half-life of 396.1 days. All of the remaining radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has at least four meta state
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
s, with the most stable being 236mNp with a half-life of 22.5 hours. .
The isotopes of neptunium range in atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 219.032 u (219Np) to 244.068 u (244Np), though 221Np has not yet been reported. Most of the isotopes that are lighter than the most stable one, 237Np, decay
Decay may refer to:
Science and technology
* Bit decay, in computing
* Decay time (fall time), in electronics
* Distance decay, in geography
* Software decay, in computing
Biology
* Decomposition of organic matter
* Mitochondrial decay, in g ...
primarily by electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Th ...
although a sizable number, most notably 229Np and 230Np, also exhibit various levels of decay via alpha emission
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an atom ...
to become protactinium
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which p ...
. 237Np itself, being the beta-stable isobar of mass number 237, decays almost exclusively by alpha emission into 233 Pa, with very rare (occurring only about once in trillions of decays) spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
and cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
(emission of 30Mg to form 207Tl). All of the known isotopes except one that are heavier than this decay exclusively via beta emission
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron t ...
. The lone exception, 240mNp, exhibits a rare (>0.12%) decay by isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
in addition to beta emission. 237Np eventually decays to form bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
-209 and thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
-205, unlike most other common heavy nuclei which decay into isotopes of lead
Lead (82Pb) has four observationally stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay ch ...
. This decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
is known as the neptunium series
In nuclear science a decay chain refers to the predictable series of radioactive decay, radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radionuclide, Radioactive isotopes do not usually decay directly ...
. This decay chain had long been extinct on Earth due to the short half-lives of all of its isotopes above bismuth-209, but is now being resurrected thanks to artificial production of neptunium on the tonne scale.
The isotopes neptunium-235, -236, and -237 are predicted to be fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissil ...
; only neptunium-237's fissionability has been experimentally shown, with the critical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
being about 60 kg, only about 10 kg more than that of the commonly used uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
. Calculated values of the critical masses of neptunium-235, -236, and -237 respectively are 66.2 kg, 6.79 kg, and 63.6 kg: the neptunium-236 value is even lower than that of plutonium-239
Plutonium-239 ( or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main iso ...
. In particular, 236Np also has a low neutron cross section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture and engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**A ...
. Despite this, a neptunium atomic bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear expl ...
has never been built: uranium and plutonium have lower critical masses than 235Np and 237Np, and 236Np is difficult to purify as it is not found in quantity in spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
and is nearly impossible to separate in any significant quantities from 237Np.
Occurrence
The longest-lived isotope of neptunium, 237Np, has a half-life of 2.14 million years, which is more than 2,000 times shorter than theage of the Earth
The age of Earth is estimated to be 4.54 ± 0.05 billion years. This age may represent the age of Earth's accretion (astrophysics), accretion, or Internal structure of Earth, core formation, or of the material from which Earth formed. This dating ...
. Therefore, any primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial soup, hypothetical conditions under which life on Earth may have begun
* Primordial nuclide, nuclides, a few radioactive, that form ...
neptunium would have decayed in the distant past. After only about 80 million years, the concentration of even the longest-lived isotope, 237Np, would have been reduced to less than one-trillionth (10−12) of its original amount.Yoshida et al., pp. 703–4. Thus neptunium is present in nature only in negligible amounts produced as intermediate decay products of other isotopes.
Trace
Trace may refer to:
Arts and entertainment Music
* ''Trace'' (Son Volt album), 1995
* ''Trace'' (Died Pretty album), 1993
* Trace (band), a Dutch progressive rock band
* ''The Trace'' (album), by Nell
Other uses in arts and entertainment
* ...
amounts of the neptunium isotopes neptunium-237 and -239 are found naturally as decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( d ...
s from transmutation reactions in uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
s.Emsley, pp. 345–347. 239Np and 237Np are the most common of these isotopes; they are directly formed from neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
by uranium-238 atoms. These neutrons come from the spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
of uranium-238, naturally neutron-induced fission of uranium-235, cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ene ...
of nuclei, and light elements absorbing alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s and emitting a neutron. The half-life of 239Np is very short, although the detection of its much longer-lived daughter
A daughter is a female offspring; a girl or a woman in relation to her parents. Daughterhood is the state, condition or quality of being someone's daughter. The male counterpart is a son. Analogously the name is used in several areas to show r ...
239Pu in nature in 1951 definitively established its natural occurrence. In 1952, 237Np was identified and isolated from concentrates of uranium ore from the Belgian Congo
The Belgian Congo (, ; ) was a Belgian colonial empire, Belgian colony in Central Africa from 1908 until independence in 1960 and became the Republic of the Congo (Léopoldville). The former colony adopted its present name, the Democratic Repu ...
: in these minerals, the ratio of neptunium-237 to uranium is less than or equal to about 10−12 to 1.
Additionally, 240Np must also occur as an intermediate decay product of 244Pu, which has been detected in meteorite dust in marine sediments on Earth.
Most neptunium (and plutonium) now encountered in the environment is due to atmospheric nuclear explosions that took place between the detonation of the first atomic bomb in 1945 and the ratification of the Partial Nuclear Test Ban Treaty
The Partial Test Ban Treaty (PTBT), formally known as the 1963 Treaty Banning Nuclear Weapon Tests in the Atmosphere, in Outer Space and Under Water, prohibited all nuclear weapons testing, test detonations of nuclear weapons except for those co ...
in 1963. The total amount of neptunium released by these explosions and the few atmospheric tests that have been carried out since 1963 is estimated to be around 2500 kg. The overwhelming majority of this is composed of the long-lived isotopes 236Np and 237Np since even the moderately long-lived 235Np (half-life 396 days) would have decayed to less than one-billionth (10−9) its original concentration over the intervening decades. An additional very small amount of neptunium, produced by neutron irradiation of natural uranium in nuclear reactor cooling water, is released when the water is discharged into rivers or lakes. The concentration of 237Np in seawater is approximately 6.5 × 10−5 millibecquerels per liter
The litre ( Commonwealth spelling) or liter ( American spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cu ...
: this concentration is between 0.1% and 1% that of plutonium.
Once released in the surface environment, in contact with atmospheric oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, neptunium generally oxidizes
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
fairly quickly, usually to the +4 or +5 state. Regardless of its oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
, the element exhibits much greater mobility than the other actinides, largely due to its ability to readily form aqueous solutions with various other elements. In one study comparing the diffusion rates of neptunium(V), plutonium(IV), and americium(III) in sandstone and limestone, neptunium penetrated more than ten times as well as the other elements. Np(V) will also react efficiently in pH levels greater than 5.5 if there are no carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s present and in these conditions it has also been observed to readily bond with quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
. It has also been observed to bond well with goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
, ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when us ...
colloids, and several clays including kaolinite
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina () ...
and smectite
A smectite (; ; ) is a mineral mixture of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secon ...
. Np(V) does not bond as readily to soil particles in mildly acidic conditions as its fellow actinides americium and curium by nearly an order of magnitude. This behavior enables it to migrate rapidly through the soil while in solution without becoming fixed in place, contributing further to its mobility.Atwood, section 4. Np(V) is also readily absorbed by concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
, which because of the element's radioactivity is a consideration that must be addressed when building nuclear waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
storage facilities. When absorbed in concrete, it is reduced
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox reacti ...
to Np(IV) in a relatively short period of time. Np(V) is also reduced by humic acid
Humic substances (HS) are colored relatively recalcitrant organic compounds naturally formed during long-term decomposition and transformation of biomass residues. The color of humic substances varies from bright yellow to light or dark brown lead ...
s if they are present on the surface of goethite, hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
, and magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
. Np(IV) is less mobile and efficiently adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
by tuff
Tuff is a type of rock made of volcanic ash ejected from a vent during a volcanic eruption. Following ejection and deposition, the ash is lithified into a solid rock. Rock that contains greater than 75% ash is considered tuff, while rock co ...
, granodiorite
Granodiorite ( ) is a coarse-grained (phaneritic) intrusive igneous rock similar to granite, but containing more plagioclase feldspar than orthoclase feldspar.
The term banatite is sometimes used informally for various rocks ranging from gra ...
, and bentonite
Bentonite ( ) is an Absorption (chemistry), absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelli ...
; although uptake by the latter is most pronounced in mildly acidic conditions. It also exhibits a strong tendency to bind to colloidal particulates, an effect that is enhanced when in surface soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
with high clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impuriti ...
content. The behavior provides an additional aid in the element's observed high mobility.Atwood, section 1.
History
Background and early claims
 When the first
When the first periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
of the elements was published by Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
in the early 1870s, it showed a " — " in place after uranium similar to several other places for then-undiscovered elements. Other subsequent tables of known elements, including a 1913 publication of the known radioactive isotopes by Kasimir Fajans
Kazimierz Fajans (Kasimir Fajans in many American publications; 27 May 1887 – 18 May 1975) was a Polish-Jewish physical chemist, a pioneer in the science of radioactivity and the co-discoverer of chemical element protactinium.
Education and ca ...
, also show an empty place after uranium, element 92.
Up to and after the discovery of the final component of the atomic nucleus, the neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
in 1932, most scientists did not seriously consider the possibility of elements heavier than uranium. While nuclear theory at the time did not explicitly prohibit their existence, there was little evidence to suggest that they did. However, the discovery of induced radioactivity
Induced radioactivity, also called artificial radioactivity or man-made radioactivity, is the process of using radiation to make a previously stable material radioactive. The husband-and-wife team of Irène Joliot-Curie and Frédéric Joliot-Curie ...
by Irène and Frédéric Joliot-Curie
Jean Frédéric Joliot-Curie (; ; 19 March 1900 – 14 August 1958) was a French chemist and physicist who received the 1935 Nobel Prize in Chemistry with his wife, Irène Joliot-Curie, for their discovery of induced radioactivity. They were t ...
in late 1933 opened up an entirely new method of researching the elements and inspired a small group of Italian scientists led by Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian and naturalized American physicist, renowned for being the creator of the world's first artificial nuclear reactor, the Chicago Pile-1, and a member of the Manhattan Project ...
to begin a series of experiments involving neutron bombardment. Although the Joliot-Curies' experiment involved bombarding a sample of 27 Al with alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s to produce the radioactive 30 P, Fermi realized that using neutrons, which have no electrical charge, would most likely produce even better results than the positively charged alpha particles. Accordingly, in March 1934 he began systematically subjecting all of the then-known elements to neutron bombardment to determine whether others could also be induced to radioactivity.
After several months of work, Fermi's group had tentatively determined that lighter elements would disperse the energy of the captured neutron by emitting a proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
or alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
and heavier elements would generally accomplish the same by emitting a gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. This latter behavior would later result in the beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of a neutron into a proton, thus moving the resulting isotope one place up the periodic table. When Fermi's team bombarded uranium, they observed this behavior as well, which strongly suggested that the resulting isotope had an atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
of 93. Fermi was initially reluctant to publicize such a claim, but after his team observed several unknown half-lives in the uranium bombardment products that did not match those of any known isotope, he published a paper entitled ''Possible Production of Elements of Atomic Number Higher than 92'' in June 1934. For element 93, he proposed the name ''ausenium
''Ausenium'' (atomic symbol Ao) and ''Hesperium'' (atomic symbol Es) were the names initially assigned to the transuranic elements with atomic numbers 93 and 94, respectively. The discovery of the elements, now discredited, was made by Enrico Fermi ...
'' (atomic symbol Ao) after the Greek name ''Ausonia'' for Italy.
Several theoretical objections to the claims of Fermi's paper were quickly raised; in particular, the exact process that took place when an atom captured a neutron was not well understood at the time. This and Fermi's accidental discovery three months later that nuclear reactions could be induced by slow neutrons cast further doubt in the minds of many scientists, notably Aristid von Grosse
Aristid von Grosse (January 1905 – July 21, 1985) was a Germans, German nuclear chemist. During his work with Otto Hahn, he got access to waste material from radium production, and with this starting material he was able in 1927 to isolate ...
and Ida Noddack
Ida Noddack (25 February 1896 – 24 September 1978), ''née'' Tacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg, she discovered ...
, that the experiment was creating element 93. While von Grosse's claim that Fermi was actually producing protactinium
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which p ...
(element 91) was quickly tested and disproved, Noddack's proposal that the uranium had been shattered into two or more much smaller fragments was simply ignored by most because existing nuclear theory did not include a way for this to be possible. Fermi and his team maintained that they were in fact synthesizing a new element, but the issue remained unresolved for several years.
Although the many different and unknown radioactive half-lives in the experiment's results showed that several nuclear reactions were occurring, Fermi's group could not prove that element 93 was being produced unless they could isolate it chemically. They and many other scientists attempted to accomplish this, including Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
and Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
who were among the best radiochemists in the world at the time and supporters of Fermi's claim, but they all failed. Much later, it was determined that the main reason for this failure was because the predictions of element 93's chemical properties were based on a periodic table which lacked the actinide series
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
. This arrangement placed protactinium below tantalum, uranium below tungsten, and further suggested that element 93, at that point referred to as eka-rhenium, should be similar to the group 7 element
Group 7, numbered by International Union of Pure and Applied Chemistry, IUPAC nomenclature, is a Group (periodic table), group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This g ...
s, including manganese and rhenium. Thorium, protactinium, and uranium, with their dominant oxidation states of +4, +5, and +6 respectively, fooled scientists into thinking they belonged below hafnium, tantalum, and tungsten, rather than below the lanthanide series, which was at the time viewed as a fluke, and whose members all have dominant +3 states; neptunium, on the other hand, has a much rarer, more unstable +7 state, with +4 and +5 being the most stable. Upon finding that plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
and the other transuranic elements also have dominant +3 and +4 states, along with the discovery of the f-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-bl ...
, the actinide series was firmly established.
While the question of whether Fermi's experiment had produced element 93 was stalemated, two additional claims of the discovery of the element appeared, although unlike Fermi, they both claimed to have observed it in nature. The first of these claims was by Czech engineer Odolen Koblic in 1934 when he extracted a small amount of material from the wash water of heated pitchblende
Uraninite, also known as pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the urani ...
. He proposed the name bohemium for the element, but after being analyzed it turned out that the sample was a mixture of tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
and vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
. The other claim, in 1938 by Romanian physicist Horia Hulubei
Horia Hulubei (; 15 November 1896 – 22 November 1972) was a Romanian nuclear physicist, known for his contributions to the development of X-ray spectroscopy.
Education and military service
Born in Iași, he graduated in 1915 first in his clas ...
and French chemist Yvette Cauchois
Yvette Cauchois (; 19 December 1908 – 19 November 1999) was a French physicist known for her contributions to X-ray spectroscopy and X-ray optics, and for pioneering European synchrotron research.
Education
Cauchois attended school in ...
, claimed to have discovered the new element via spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
in minerals. They named their element sequanium Sequanium was the proposed name for a new element that Romanian physicist Horia Hulubei reported he had discovered in 1939. The name derived from the Latin word Sequana for the river Seine running through Paris where Hulubei worked at that time.
Hu ...
, but the claim was discounted because the prevailing theory at the time was that if it existed at all, element 93 would not exist naturally. However, as neptunium does in fact occur in nature in trace amounts, as demonstrated when it was found in uranium ore in 1952, it is possible that Hulubei and Cauchois did in fact observe neptunium.
Although by 1938 some scientists, including Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, were still reluctant to accept that Fermi had actually produced a new element, he was nevertheless awarded the Nobel Prize in Physics
The Nobel Prize in Physics () is an annual award given by the Royal Swedish Academy of Sciences for those who have made the most outstanding contributions to mankind in the field of physics. It is one of the five Nobel Prizes established by the ...
in November 1938 "for his demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons". A month later, the almost totally unexpected discovery of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
by Hahn, Meitner, and Otto Frisch
Otto Robert Frisch (1 October 1904 – 22 September 1979) was an Austrian-born British physicist who worked on nuclear physics. With Otto Stern and Immanuel Estermann, he first measured the magnetic moment of the proton. With his aunt, Lise M ...
put an end to the possibility that Fermi had discovered element 93 because most of the unknown half-lives that had been observed by Fermi's team were rapidly identified as those of fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
.
Perhaps the closest of all attempts to produce the missing element 93 was that conducted by the Japanese physicist Yoshio Nishina
was a Japanese physicist who was called "the founding father of modern physics research in Japan". He led the efforts of Japan to develop an atomic bomb during World War II.
Early life and career
Nishina was born in Satoshō, Okayama. He rece ...
working with chemist Kenjiro Kimura in 1940, just before the outbreak of the Pacific War
The Pacific War, sometimes called the Asia–Pacific War or the Pacific Theatre, was the Theater (warfare), theatre of World War II fought between the Empire of Japan and the Allies of World War II, Allies in East Asia, East and Southeast As ...
in 1941: they bombarded 238U with fast neutrons. However, while slow neutrons tend to induce neutron capture through a (n, γ) reaction, fast neutrons tend to induce a "knock-out" (n, 2n) reaction, where one neutron is added and two more are removed, resulting in the net loss of a neutron. Nishina and Kimura, having tested this technique on 232 Th and successfully produced the known 231Th and its long-lived beta decay daughter 231 Pa (both occurring in the natural decay chain of 235U), therefore correctly assigned the new 6.75-day half-life activity they observed to the new isotope 237U. They confirmed that this isotope was also a beta emitter and must hence decay to the unknown nuclide 23793. They attempted to isolate this nuclide by carrying it with its supposed lighter congener rhenium, but no beta or alpha decay was observed from the rhenium-containing fraction: Nishina and Kimura thus correctly speculated that the half-life of 23793, like that of 231Pa, was very long and hence its activity would be so weak as to be unmeasurable by their equipment, thus concluding the last and closest unsuccessful search for transuranic elements.
Discovery
 As research on nuclear fission progressed in early 1939,
As research on nuclear fission progressed in early 1939, Edwin McMillan
Edwin Mattison McMillan (September 18, 1907 – September 7, 1991) was an American physicist credited with being the first to produce a transuranium element, neptunium. For this, he shared the 1951 Nobel Prize in Chemistry with Glenn Seaborg.
...
at the Berkeley Radiation Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a federally funded research and development center in the hills of Berkeley, California, United States. Established in 1931 by the University of California (UC), the laboratory is spo ...
of the University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California), is a Public university, public Land-grant university, land-grant research university in Berkeley, California, United States. Founded in 1868 and named after t ...
decided to run an experiment bombarding uranium using the powerful 60-inch (1.52 m) cyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
that had recently been built at the university. The purpose was to separate the various fission products produced by the bombardment by exploiting the enormous force that the fragments gain from their mutual electrical repulsion after fissioning. Although he did not discover anything of note from this, McMillan did observe two new beta decay half-lives in the uranium trioxide target itself, which meant that whatever was producing the radioactivity had not violently repelled each other like normal fission products. He quickly realized that one of the half-lives closely matched the known 23-minute decay period of uranium-239, but the other half-life of 2.3 days was unknown. McMillan took the results of his experiment to chemist and fellow Berkeley professor Emilio Segrè
Emilio Gino Segrè ( ; ; 1 February 1905 – 22 April 1989) was an Italian-American nuclear physicist and radiochemist who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was award ...
to attempt to isolate the source of the radioactivity. Both scientists began their work using the prevailing theory that element 93 would have similar chemistry to rhenium, but Segrè rapidly determined that McMillan's sample was not at all similar to rhenium. Instead, when he reacted it with hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
(HF) with a strong oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
present, it behaved much like members of the rare earths
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of ...
. Since these elements comprise a large percentage of fission products, Segrè and McMillan decided that the half-life must have been simply another fission product, titling the paper "An Unsuccessful Search for Transuranium Elements".
However, as more information about fission became available, the possibility that the fragments of nuclear fission could still have been present in the target became more remote. McMillan and several scientists, including Philip H. Abelson, attempted again to determine what was producing the unknown half-life. In early 1940, McMillan realized that his 1939 experiment with Segrè had failed to test the chemical reactions of the radioactive source with sufficient rigor. In a new experiment, McMillan tried subjecting the unknown substance to HF in the presence of a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
, something he had not done before. This reaction resulted in the sample precipitating with the HF, an action that definitively ruled out the possibility that the unknown substance was a rare-earth metal.
Shortly after this, Abelson, who had received his graduate degree
Postgraduate education, graduate education, or graduate school consists of academic or professional degrees, certificates, diplomas, or other qualifications usually pursued by post-secondary students who have earned an undergraduate (bachelor ...
from the university, visited Berkeley for a short vacation and McMillan asked the more able chemist to assist with the separation of the experiment's results. Abelson very quickly observed that whatever was producing the 2.3-day half-life did not have chemistry like any known element and was actually more similar to uranium than a rare-earth metal. This discovery finally allowed the source to be isolated and later, in 1945, led to the classification of the actinide series
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
. As a final step, McMillan and Abelson prepared a much larger sample of bombarded uranium that had a prominent 23-minute half-life from 239U and demonstrated conclusively that the unknown 2.3-day half-life increased in strength in concert with a decrease in the 23-minute activity through the following reaction:
:Physical Review
''Physical Review'' is a peer-reviewed scientific journal. The journal was established in 1893 by Edward Nichols. It publishes original research as well as scientific and literature reviews on all aspects of physics. It is published by the Ame ...
'' on May 27, 1940. They did not propose a name for the element in the paper, but they soon decided on the name ''neptunium'' since Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
is the next planet beyond Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
in the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
, which uranium is named after. McMillan and Abelson's success compared to Nishina and Kimura's near miss can be attributed to the favorable half-life of 239Np for radiochemical analysis and quick decay of 239U, in contrast to the slower decay of 237U and extremely long half-life of 237Np.
Subsequent developments
It was also realized that the beta decay of 239Np must produce an isotope of element 94 (now calledplutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
), but the quantities involved in McMillan and Abelson's original experiment were too small to isolate and identify plutonium along with neptunium. The discovery of plutonium had to wait until the end of 1940, when Glenn T. Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
and his team identified the isotope plutonium-238
Plutonium-238 ( or Pu-238) is a radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suitable for usage ...
.
In 1942, Hahn and Fritz Strassmann
Friedrich Wilhelm Strassmann (; 22 February 1902 – 22 April 1980) was a German chemist who, with Otto Hahn in December 1938, identified the element barium as a product of the bombardment of uranium with neutrons. Their observation was the key ...
, and independently Kurt Starke
Kurt Starke (1911 in Berlin – 19 January 2000) was a German radiochemist. During World War II, he worked on the German nuclear energy project, also known as the Uranium Club. He independently discovered the transuranic element neptunium. From ...
, reported the confirmation of element 93 in Berlin. Hahn's group did not pursue element 94, likely because they were discouraged by McMillan and Abelson's lack of success in isolating it. Since they had access to the stronger cyclotron at Paris at this point, Hahn's group would likely have been able to detect element 94 had they tried, albeit in tiny quantities (a few becquerels
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as an activity of one per second, on average, for aperiodic activity events referred to a radionuclide. For applicatio ...
).
Neptunium's unique radioactive characteristics allowed it to be traced as it moved through various compounds in chemical reactions, at first this was the only method available to prove that its chemistry was different from other elements. As the first isotope of neptunium to be discovered has such a short half-life, McMillan and Abelson were unable to prepare a sample that was large enough to perform chemical analysis of the new element using the technology that was then available. However, after the discovery of the long-lived 237Np isotope in 1942 by Glenn Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
and Arthur Wahl
Arthur Charles Wahl (September 8, 1917 – March 6, 2006) was an American chemist who, as a doctoral student of Glenn T. Seaborg at the University of California, Berkeley, first isolated plutonium (94) in February 1941 shortly after the elem ...
, forming weighable amounts of neptunium became a realistic endeavor. Its half-life was initially determined to be about 3 million years (later revised to 2.144 million years), confirming the predictions of Nishina and Kimura of a very long half-life.
Early research into the element was somewhat limited because most of the nuclear physicists and chemists in the United States at the time were focused on the massive effort to research the properties of plutonium as part of the Manhattan Project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada.
From 1942 to 1946, the ...
. Research into the element did continue as a minor part of the project and the first bulk sample of neptunium (as neptunium dioxide
Neptunium(IV) oxide, or neptunium dioxide, is a radioactive, olive green cubic crystalline solid with the formula NpO2. It is one of two stable oxides of neptunium, the other being neptunium(V) oxide. It emits both α- and γ-particles.
Produc ...
) was isolated in 1944.
Much of the research into the properties of neptunium since then has been focused on understanding how to confine it as a portion of nuclear waste. Because it has isotopes with very long half-lives, it is of particular concern in the context of designing confinement facilities that can last for thousands of years. It has found some limited uses as a radioactive tracer and a precursor for various nuclear reactions to produce useful plutonium isotopes. However, most of the neptunium that is produced as a reaction byproduct in nuclear power stations is considered to be a waste product.
Production

Synthesis
The vast majority of the neptunium that currently exists on Earth was produced artificially in nuclear reactions. Neptunium-237 is the most commonly synthesized isotope due to it being the only one that both can be produced vianeutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
and also has a half-life long enough to allow weighable quantities to be easily isolated. It is by far the most common isotope to be utilized in chemical studies of the element.Yoshida et al., p. 700–2.
* When an 235U atom captures a neutron, it is converted to an excited state of 236U. About 85.5% of the excited 236U nuclei undergo fission, but the remainder decay to the ground state of 236U by emitting gamma radiation
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. Further neutron capture forms 237U which has a half-life of 7 days and quickly decays to 237Np through beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
. During beta decay, the excited 237U emits an electron, while the atomic weak interaction
In nuclear physics and particle physics, the weak interaction, weak force or the weak nuclear force, is one of the four known fundamental interactions, with the others being electromagnetism, the strong interaction, and gravitation. It is th ...
converts a neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
to a proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
, thus creating 237Np.
::
* 237U is also produced via an ( n,2n) reaction with 238U. This only happens with very energetic neutrons.
* 237Np is the product of alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of 241Am, which is produced through neutron irradiation of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
.
Heavier isotopes of neptunium decay quickly, and lighter isotopes of neptunium cannot be produced by neutron capture, so chemical separation of neptunium from cooled spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
gives nearly pure 237Np. The short-lived heavier isotopes 238Np and 239Np, useful as radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
s, are produced through neutron irradiation of 237Np and 238U respectively, while the longer-lived lighter isotopes 235Np and 236Np are produced through irradiation of 235U with proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and deuteron
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium atomic nucleus, nucleus (deuteron) contains one proton and ...
s in a cyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
.
Artificial 237Np metal is usually isolated through a reaction of 237NpF3 with liquid barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
or lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
at around 1200 ° C and is most often extracted from spent nuclear fuel rod
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other atomic nucleus, nuclear devices to generate energy.
Oxide fuel
For fission reactors, the fuel (typically based on uranium) is ...
s in kilogram amounts as a by-product in plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
production.
:2 NpF3 + 3 Ba → 2 Np + 3 BaF2
By weight, neptunium-237 discharges are about 5% as great as plutonium discharges and about 0.05% of spent nuclear fuel discharges. However, even this fraction still amounts to more than fifty tons per year globally.
Purification methods
Recovering uranium and plutonium from spent nuclear fuel for reuse is one of the major processes of thenuclear fuel cycle
The nuclear fuel cycle, also known as the nuclear fuel chain, describes the series of stages that nuclear fuel undergoes during its production, use, and recycling or disposal. It consists of steps in the ''front end'', which are the preparation o ...
. As it has a long half-life of just over 2 million years, the alpha emitter
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produced ...
237Np is one of the major isotopes of the minor actinide
Minor may refer to:
Common meanings
* Minor (law), a person not under the age of certain legal activities.
* Academic minor, a secondary field of study in undergraduate education
Mathematics
* Minor (graph theory), a relation of one graph to ...
s separated from spent nuclear fuel.Yodshida et al., pp. 704–5. Many separation methods have been used to separate out the neptunium, operating on small and large scales. The small-scale purification operations have the goals of preparing pure neptunium as a precursor
Precursor or Precursors may refer to:
*Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), hypothesized genus of fossil birds that was composed of fossilized parts of unre ...
of metallic neptunium and its compounds, and also to isolate and preconcentrate neptunium in samples for analysis.
Most methods that separate neptunium ions exploit the differing chemical behaviour of the differing oxidation states of neptunium (from +3 to +6 or sometimes even +7) in solution. Among the methods that are or have been used are: solvent extraction Extraction may refer to:
Science and technology
Biology and medicine
* Comedo extraction, a method of acne treatment
* Dental extraction, the surgical removal of a tooth from the mouth
Computing and information science
* Data extraction, the ...
(using various extractant
Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly ho ...
s, usually multidentate β-diketone derivatives, organophosphorus compound
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbo ...
s, and amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
compounds), chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
using various ion-exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
or chelating
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
resins, coprecipitation
In chemistry, coprecipitation (CPT) or co-precipitation is the carrying down by a precipitate of substances normally soluble under the conditions employed. Analogously, in medicine, coprecipitation (referred to as immunoprecipitation) is specific ...
(possible matrices
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the ...
include LaF3, BiPO4, BaSO4, Fe(OH)3, and MnO2), electrodeposition, and biotechnological
Biotechnology is a multidisciplinary field that involves the integration of natural sciences and engineering sciences in order to achieve the application of organisms and parts thereof for products and services. Specialists in the field are kn ...
methods.Yoshida et al., pp. 705–17. Currently, commercial reprocessing plants use the Purex process, involving the solvent extraction of uranium and plutonium with tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric a ...
.Yoshida et al., p. 710.
Chemistry and compounds
Solution chemistry
 When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of
When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of oxidizing
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
or reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s, pH of the solution, presence of coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
-forming ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s, and even the concentration of neptunium in the solution.Yoshida et al., pp. 752–4.
In acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
solutions, the neptunium(III) to neptunium(VII) ions exist as Np3+, Np4+, , , and . In basic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
solutions, they exist as the oxides and hydroxides Np(OH)3, NpO2, NpO2OH, NpO2(OH)2, and . Not as much work has been done to characterize neptunium in basic solutions. Np3+ and Np4+ can easily be reduced and oxidized to each other, as can and .Yoshida et al., p. 759.
;Neptunium(III)
Np(III) or Np3+ exists as hydrated complexes in acidic solutions, . It is a dark blue-purple and is analogous to its lighter congener, the pink rare-earth
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of ...
ion Pm3+. In the presence of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, it is quickly oxidized to Np(IV) unless strong reducing agents are also present. Nevertheless, it is the second-least easily hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
neptunium ion in water, forming the NpOH2+ ion. Np3+ is the predominant neptunium ion in solutions of pH 4–5.Yoshida et al., p. 766–70.
;Neptunium(IV)
Np(IV) or Np4+ is pale yellow-green in acidic solutions, where it exists as hydrated complexes (). It is quite unstable to hydrolysis in acidic aqueous solutions at pH 1 and above, forming NpOH3+. In basic solutions, Np4+ tends to hydrolyze to form the neutral neptunium(IV) hydroxide (Np(OH)4) and neptunium(IV) oxide (NpO2).
;Neptunium(V)
Np(V) or is green-blue in aqueous solution, in which it behaves as a strong Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
. It is a stable ion and is the most common form of neptunium in aqueous solutions. Unlike its neighboring homologues and , does not spontaneously disproportionate
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
except at very low pH and high concentration:
:2 + 4 H+ ⇌ Np4+ + + 2 H2O
It hydrolyzes in basic solutions to form NpO2OH and .
;Neptunium(VI)
Np(VI) or , the neptunyl ion, shows a light pink or reddish color in an acidic solution and yellow-green otherwise. It is a strong Lewis acid and is the main neptunium ion encountered in solutions of pH 3–4. Though stable in acidic solutions, it is quite easily reduced to the Np(V) ion, and it is not as stable as the homologous hexavalent ions of its neighbours uranium and plutonium (the uranyl
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the u ...
and plutonyl
The plutonyl ion is an oxycation of plutonium in the oxidation state +6, with the chemical formula . It is isostructural with the uranyl ion, compared to which it has a slightly shorter M–O bond. It is easily reduced to plutonium(III). The pluto ...
ions). It hydrolyzes in basic solutions to form the oxo and hydroxo ions NpO2OH+, , and .
;Neptunium(VII)
Np(VII) is dark green in a strongly basic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
solution. Though its chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
in basic solution is frequently cited as , this is a simplification and the real structure is probably closer to a hydroxo species like . Np(VII) was first prepared in basic solution in 1967. In strongly acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
solution, Np(VII) is found as ; water quickly reduces this to Np(VI). Its hydrolysis products are uncharacterized.
Hydroxides
The oxides and hydroxides of neptunium are closely related to its ions. In general, Np hydroxides at various oxidation levels are less stable than the actinides before it on the periodic table such asthorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
and uranium and more stable than those after it such as plutonium and americium. This phenomenon is because the stability of an ion increases as the ratio of atomic number to the radius of the ion increases. Thus actinides higher on the periodic table will more readily undergo hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
.
Neptunium(III) hydroxide is quite stable in acidic solutions and in environments that lack oxygen, but it will rapidly oxidize to the IV state in the presence of air. It is not soluble in water. Np(IV) hydroxides exist mainly as the electrically neutral Np(OH)4 and its mild solubility in water is not affected at all by the pH of the solution. This suggests that the other Np(IV) hydroxide, , does not have a significant presence.
Because the Np(V) ion is very stable, it can only form a hydroxide in high acidity levels. When placed in a 0.1 M sodium perchlorate
Sodium perchlorate is an inorganic compound with the chemical formula . It consists of sodium cations and perchlorate anions . It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as s ...
solution, it does not react significantly for a period of months, although a higher molar concentration of 3.0 M will result in it reacting to the solid hydroxide NpO2OH almost immediately. Np(VI) hydroxide is more reactive but it is still fairly stable in acidic solutions. It will form the compound NpO3· H2O in the presence of ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
under various carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
pressures. Np(VII) has not been well-studied and no neutral hydroxides have been reported. It probably exists mostly as .
Oxides
Three anhydrous neptunium oxides have been reported, NpO2, Np2O5, and Np3O8, though some studies have stated that only the first two of these exist, suggesting that claims of Np3O8 are actually the result of mistaken analysis of Np2O5. However, as the full extent of the reactions that occur between neptunium and oxygen has yet to be researched, it is not certain which of these claims is accurate. Although neptunium oxides have not been produced with neptunium in oxidation states as high as those possible with the adjacent actinide uranium, neptunium oxides are more stable at lower oxidation states. This behavior is illustrated by the fact that NpO2 can be produced by simply burning neptunium salts of oxyacids in air.Yoshida et al., 724–726. The greenish-brown NpO2 is very stable over a large range of pressures and temperatures and does not undergophase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s at low temperatures. It does show a phase transition from face-centered cubic to orthorhombic at around 33–37 GPa, although it returns to its original phase when pressure is released. It remains stable under oxygen pressures up to 2.84 MPa and temperatures up to 400 °C.
Np2O5 is black-brown in color and monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in t ...
with a lattice size of 418×658×409 picometres. It is relatively unstable and decomposes to NpO2 and O2 at 420–695 °C. Although Np2O5 was initially subject to several studies that claimed to produce it with mutually contradictory methods, it was eventually prepared successfully by heating neptunium peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
to 300–350 °C for 2–3 hours or by heating it under a layer of water in an ampoule
An ampoule (also ampul and ampule) is a small sealed vial which is used to contain and preserve a sample, usually a solid or liquid. Ampoules are usually made of glass.
Modern ampoules are most commonly used to contain pharmaceuticals and chem ...
at 180 °C.
Neptunium also forms a large number of oxide compounds with a wide variety of elements, although the neptunate oxides formed with alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s and alkaline earth metals
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are a ...
have been by far the most studied. Ternary neptunium oxides are generally formed by reacting NpO2 with the oxide of another element or by precipitating from an alkaline solution. Li5NpO6 has been prepared by reacting Li2O and NpO2 at 400 °C for 16 hours or by reacting Li2O2 with NpO3 · H2O at 400 °C for 16 hours in a quartz tube and flowing oxygen. Alkali neptunate compounds K3NpO5, Cs3NpO5, and Rb3NpO5 are all produced by a similar reaction:
:NpO2 + 3 MO2 → M3NpO5 (M = K, Cs, Rb)
The oxide compounds KNpO4, CsNpO4, and RbNpO4 are formed by reacting Np(VII) () with a compound of the alkali metal nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
and ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
. Additional compounds have been produced by reacting NpO3 and water with solid alkali and alkaline peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s at temperatures of 400–600 °C for 15–30 hours. Some of these include Ba3(NpO5)2, Ba2 NaNpO6, and Ba2LiNpO6. Also, a considerable number of hexavalent neptunium oxides are formed by reacting solid-state NpO2 with various alkali or alkaline earth oxides in an environment of flowing oxygen. Many of the resulting compounds also have an equivalent compound that substitutes uranium for neptunium. Some compounds that have been characterized include Na2Np2O7, Na4NpO5, Na6NpO6, and Na2NpO4. These can be obtained by heating different combinations of NpO2 and Na2O to various temperature thresholds and further heating will also cause these compounds to exhibit different neptunium allotropes. The lithium neptunate oxides Li6NpO6 and Li4NpO5 can be obtained with similar reactions of NpO2 and Li2O.Yoshida et al, pp. 728–730.
A large number of additional alkali and alkaline neptunium oxide compounds such as Cs4Np5O17 and Cs2Np3O10 have been characterized with various production methods. Neptunium has also been observed to form ternary oxides with many additional elements in group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
s 3 through 7, although these compounds are much less well studied.
Halides
Although neptuniumhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
compounds have not been nearly as well studied as its oxides, a fairly large number have been successfully characterized. Of these, neptunium fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
s have been the most extensively researched, largely because of their potential use in separating the element from nuclear waste products. Four binary neptunium fluoride compounds, Np F3, NpF4, NpF5, and NpF6, have been reported. The first two are fairly stable and were first prepared in 1947 through the following reactions:
:NpO2 + H2 + 3 HF → NpF3 + 2 H2O (400°C)
:NpF3 + O2 + HF → NpF4 + H2O (400°C)
Later, NpF4 was obtained directly by heating NpO2 to various temperatures in mixtures of either hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
or pure fluorine gas. NpF5 is much more difficult to form and most known preparation methods involve reacting NpF4 or NpF6 compounds with various other fluoride compounds. NpF5 will decompose into NpF4 and NpF6 when heated to around 320 °C.Yoshida et al, pp. 730–736.
NpF6 or neptunium hexafluoride
Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is a volatile orange crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioac ...
is extremely volatile, as are its adjacent actinide compounds uranium hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons.
Preparation
Uranium dioxide is co ...
(UF6) and plutonium hexafluoride (PuF6). This volatility has attracted a large amount of interest to the compound in an attempt to devise a simple method for extracting neptunium from spent nuclear power station fuel rods. NpF6 was first prepared in 1943 by reacting NpF3 and gaseous fluorine at very high temperatures and the first bulk quantities were obtained in 1958 by heating NpF4 and dripping pure fluorine on it in a specially prepared apparatus. Additional methods that have successfully produced neptunium hexafluoride include reacting BrF3 and BrF5 with NpF4 and by reacting several different neptunium oxide and fluoride compounds with anhydrous hydrogen fluorides.
Four neptunium oxyfluoride
In chemistry, oxohalides or oxyhalides are a group of chemical compounds with the chemical formula , where X is a halogen, and A is an element different than O and X. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine ( ...
compounds, NpO2F, NpOF3, NpO2F2, and NpOF4, have been reported, although none of them have been extensively studied. NpO2F2 is a pinkish solid and can be prepared by reacting NpO3 · H2O and Np2F5 with pure fluorine at around 330 °C. NpOF3 and NpOF4 can be produced by reacting neptunium oxides with anhydrous hydrogen fluoride at various temperatures. Neptunium also forms a wide variety of fluoride compounds with various elements. Some of these that have been characterized include CsNpF6, Rb2NpF7, Na3NpF8, and K3NpO2F5.
Two neptunium chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
s, Np Cl3 and NpCl4, have been characterized. Although several attempts to obtain NpCl5 have been made, they have not been successful. NpCl3 is produced by reducing neptunium dioxide with hydrogen and carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
( CCl4) and NpCl4 by reacting a neptunium oxide with CCl4 at around 500 °C. Other neptunium chloride compounds have also been reported, including NpOCl2, Cs2NpCl6, Cs3NpO2Cl4, and Cs2NaNpCl6. Neptunium bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
s Np Br3 and NpBr4 have also been produced; the latter by reacting aluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as al ...
with NpO2 at 350 °C and the former in an almost identical procedure but with zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
present. The neptunium iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
Np I3 has also been prepared by the same method as NpBr3.Yoshida et al, pp. 736–738.
Chalcogenides, pnictides, and carbides
Neptuniumchalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
and pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
compounds have been well studied primarily as part of research into their electronic and magnetic properties and their interactions in the natural environment. Pnictide and carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
compounds have also attracted interest because of their presence in the fuel of several advanced nuclear reactor designs, although the latter group has not had nearly as much research as the former.Yoshida et al, pp. 739–742.
;Chalcogenides
A wide variety of neptunium sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
compounds have been characterized, including the pure sulfide compounds Np S, NpS3, Np2S5, Np3S5, Np2S3, and Np3S4. Of these, Np2S3, prepared by reacting NpO2 with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
at around 1000 °C, is the most well-studied and three allotropic forms are known. The α form exists up to around 1230 °C, the β up to 1530 °C, and the γ form, which can also exist as Np3S4, at higher temperatures. NpS can be produced by reacting Np2S3 and neptunium metal at 1600 °C and Np3S5 can be prepared by the decomposition of Np2S3 at 500 °C or by reacting sulfur and neptunium hydride at 650 °C. Np2S5 is made by heating a mixture of Np3S5 and pure sulfur to 500 °C. All of the neptunium sulfides except for the β and γ forms of Np2S3 are isostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
with the equivalent uranium sulfide and several, including NpS, α−Np2S3, and β−Np2S3 are also isostructural with the equivalent plutonium sulfide. The oxysulfides NpOS, Np4O4S, and Np2O2S have also been produced, although the latter three have not been well studied. NpOS was first prepared in 1985 by vacuum sealing NpO2, Np3S5, and pure sulfur in a quartz tube and heating it to 900 °C for one week.
Neptunium selenide
A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.
Inorganic selenides
Th ...
compounds that have been reported include Np Se, NpSe3, Np2Se3, Np2Se5, Np3Se4, and Np3Se5. All of these have only been obtained by heating neptunium hydride and selenium metal to various temperatures in a vacuum for an extended period of time and Np2Se3 is only known to exist in the γ allotrope at relatively high temperatures. Two neptunium oxyselenide
Oxyselenides are a group of chemical compounds that contain oxygen and selenium atoms (Figure 1). Oxyselenides can form a wide range of structures in compounds containing various transition metals, and thus can exhibit a wide range of properties. ...
compounds are known, NpOSe and Np2O2Se, are formed with similar methods by replacing the neptunium hydride with neptunium dioxide. The known neptunium telluride compounds Np Te, NpTe3, Np3Te4, Np2Te3, and Np2O2Te are formed by similar procedures to the selenides and Np2O2Te is isostructural to the equivalent uranium and plutonium compounds. No neptunium−polonium
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tel ...
compounds have been reported.
;Pnictides and carbides
Neptunium nitride
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3−, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitr ...
(Np N) was first prepared in 1953 by reacting neptunium hydride and ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
gas at around 750 °C in a quartz capillary tube. Later, it was produced by reacting different mixtures of nitrogen and hydrogen with neptunium metal at various temperatures. It has also been produced by the reduction of neptunium dioxide with diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
nitrogen gas at 1550 °C. NpN is isomorphous
In mathematics, an isomorphism is a structure-preserving mapping or morphism between two structures of the same type that can be reversed by an inverse mapping. Two mathematical structures are isomorphic if an isomorphism exists between them. ...
with uranium mononitride (UN) and plutonium mononitride (PuN) and has a melting point of 2830 °C under a nitrogen pressure of around 1 MPa. Two neptunium phosphide
In chemistry, a phosphide is a compound containing the ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of pho ...
compounds have been reported, Np P and Np3P4. The first has a face centered cubic structure and is prepared by converting neptunium metal to a powder and then reacting it with phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
gas at 350 °C. Np3P4 can be produced by reacting neptunium metal with red phosphorus
Red phosphorus is an Allotropes of phosphorus, allotrope of phosphorus. It is an amorphous polymeric red solid that is stable in air. It can be easily converted from white phosphorus under light or heating. It finds applications as matches and fir ...
at 740 °C in a vacuum and then allowing any extra phosphorus to sublimate away. The compound is non-reactive with water but will react with nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
to produce Np(IV) solution.Yoshida et al, pp. 742–744.
Three neptunium arsenide
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many Stoichiometry, stoichiometries, and in t ...
compounds have been prepared, Np As, NpAs2, and Np3As4. The first two were first produced by heating arsenic and neptunium hydride in a vacuum-sealed tube for about a week. Later, NpAs was also made by confining neptunium metal and arsenic in a vacuum tube, separating them with a quartz membrane, and heating them to just below neptunium's melting point of 639 °C, which is slightly higher than the arsenic's sublimation point of 615 °C. Np3As4 is prepared by a similar procedure using iodine as a transporting agent. NpAs2 crystals are brownish gold and Np3As4 is black. The neptunium antimonide
Antimonides (sometimes called stibnides or stibinides) are compounds of antimony with more electropositive elements. The antimonide ion is but the term refers also to any anionic derivative of antimony.
Antimonides are often prepared by heating ...
compound Np Sb was produced in 1971 by placing equal quantities of both elements in a vacuum tube, heating them to the melting point of antimony, and then heating it further to 1000 °C for sixteen days. This procedure also produced trace amounts of an additional antimonide compound Np3Sb4. One neptunium-bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
compound, NpBi, has also been reported.
The neptunium carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s Np C, Np2C3, and NpC2 (tentative) have been reported, but have not characterized in detail despite the high importance and utility of actinide carbides as advanced nuclear reactor fuel. NpC is a non-stoichiometric compound
Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in s ...
, and could be better labelled as NpC''x'' (0.82 ≤ ''x'' ≤ 0.96). It may be obtained from the reaction of neptunium hydride with graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
at 1400 °C or by heating the constituent elements together in an electric arc furnace
An electric arc furnace (EAF) is a Industrial furnace, furnace that heats material by means of an electric arc.
Industrial arc furnaces range in size from small units of approximately one-tonne capacity (used in foundry, foundries for producin ...
using a tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
electrode. It reacts with excess carbon to form pure Np2C3. NpC2 is formed from heating NpO2 in a graphite crucible at 2660–2800 °C.
Other inorganic
;Hydrides Neptunium reacts withhydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
in a similar manner to its neighbor plutonium, forming the hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
s NpH2+''x'' (face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
) and NpH3 (hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
). These are isostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
with the corresponding plutonium hydrides, although unlike PuH2+''x'', the lattice parameter
A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has ...
s of NpH2+''x'' become greater as the hydrogen content (''x'') increases. The hydrides require extreme care in handling as they decompose in a vacuum at 300 °C to form finely divided neptunium metal, which is pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
.Yoshida et al., pp. 722–4.
;Phosphates, sulfates, and carbonates
Being chemically stable, neptunium phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s have been investigated for potential use in immobilizing nuclear waste. Neptunium pyrophosphate (α-NpP2O7), a green solid, has been produced in the reaction between neptunium dioxide and boron phosphate
Boron phosphate is an inorganic compound with the chemical formula BPO4. The simplest way of producing it is the reaction of phosphoric acid and boric acid. It is a white infusible solid that evaporates above 1450 °C.Corbridge DEC 2013, '' ...
at 1100 °C, though neptunium(IV) phosphate has so far remained elusive. The series of compounds NpM2(PO4)3, where M is an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
(lithium, Li, sodium, Na, potassium, K, rubidium, Rb, or caesium, Cs), are all known. Some neptunium sulfates have been characterized, both aqueous and solid and at various oxidation states of neptunium (IV through VI have been observed). Additionally, neptunium carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s have been investigated to achieve a better understanding of the behavior of neptunium in geological repository, geological repositories and the environment, where it may come into contact with carbonate and bicarbonate aqueous solutions and form soluble complexes.Yoshida et al., pp. 744–5.
Organometallic
 A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and water than plutonium, Pu(C5H5)3 and americium, Am(C5H5)3. Other Np(IV) cyclopentadienyl compounds are known for many ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s: they have the general formula (C5H5)3NpL, where L represents a ligand.
Neptunocene, Np(C8H8)2, was synthesized in 1970 by reacting neptunium(IV) chloride with K2(C8H8). It is isomorphous
In mathematics, an isomorphism is a structure-preserving mapping or morphism between two structures of the same type that can be reversed by an inverse mapping. Two mathematical structures are isomorphic if an isomorphism exists between them. ...
to uranocene and plutonocene, and they behave chemically identically: all three compounds are insensitive to water or dilute bases but are sensitive to air, reacting quickly to form oxides, and are only slightly soluble in benzene and toluene. Other known neptunium cyclooctatetraenyl derivatives include Np(RC8H7)2 (R = ethanol, butanol) and KNp(C8H8)·2THF, which is isostructural to the corresponding plutonium compound. In addition, neptunium hydrocarbyls have been prepared, and solvated triiodide complexes of neptunium are a precursor to many organoneptunium and inorganic neptunium compounds.
Coordination complexes
There is much interest in the coordination chemistry of neptunium, because its five oxidation states all exhibit their own distinctive chemical behavior, and the coordination chemistry of the actinides is heavily influenced by the actinide contraction (the greater-than-expected decrease in ionic radii across the actinide series, analogous to the lanthanide contraction).Yoshida et al., pp. 745–750.Solid state
Few neptunium(III) coordination compounds are known, because Np(III) is readily oxidized by atmospheric oxygen while in aqueous solution. However, sodium formaldehyde sulfoxylate can reduce Np(IV) to Np(III), stabilizing the lower oxidation state and forming various sparingly soluble Np(III) coordination complexes, such as ·11H2O, ·H2O, and . Many neptunium(IV) coordination compounds have been reported, the first one being , which is isostructural with the analogous uranium(IV) coordination compound. Other Np(IV) coordination compounds are known, some involving other metals such as cobalt (·8H2O, formed at 400 K) and copper (·6H2O, formed at 600 K). Complex nitrate compounds are also known: the experimenters who produced them in 1986 and 1987 obtained single crystals by slow evaporation of the Np(IV) solution at ambient temperature in concentratednitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and excess 2,2′-pyrimidine.
The coordination chemistry of neptunium(V) has been extensively researched due to the presence of cation–cation interactions in the solid state, which had been already known for actinyl ions. Some known such compounds include the neptunyl dimer (chemistry), dimer ·8H2O and neptunium glycolate, both of which form green crystals.
Neptunium(VI) compounds range from the simple oxalate (which is unstable, usually becoming Np(IV)) to such complicated compounds as the green . Extensive study has been performed on compounds of the form , where M represents a monovalent cation and An is either uranium, neptunium, or plutonium.
Since 1967, when neptunium(VII) was discovered, some coordination compounds with neptunium in the +7 oxidation state have been prepared and studied. The first reported such compound was initially characterized as ·''n''H2O in 1968, but was suggested in 1973 to actually have the formula ·2H2O based on the fact that Np(VII) occurs as in aqueous solution. This compound forms dark green prismatic crystals with maximum edge length 0.15–0.4 millimeter, mm.
In aqueous solution
Most neptuniumcoordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es known in solution involve the element in the +4, +5, and +6 oxidation states: only a few studies have been done on neptunium(III) and (VII) coordination complexes.Yoshida et al., pp. 771–82. For the former, NpX2+ and (X = Cl, Br) were obtained in 1966 in concentrated lithium chloride, LiCl and lithium bromide, LiBr solutions, respectively: for the latter, 1970 experiments discovered that the ion could form sulfate complexes in acidic solutions, such as and ; these were found to have higher equilibrium constant, stability constants than the neptunyl ion (). A great many complexes for the other neptunium oxidation states are known: the inorganic ligands involved are the halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s, iodate, azide, nitride
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3−, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitr ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
, thiocyanate, sulfate, carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
, Chromate ion, chromate, and phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
. Many organic ligands are known to be able to be used in neptunium coordination complexes: they include acetate, propionate, glycolate, lactic acid, lactate, oxalate, malonate, phthalate, mellitate, and citrate.
Analogously to its neighbours, uranium and plutonium, the order of the neptunium ions in terms of complex formation ability is Np4+ > ≥ Np3+ > . (The relative order of the middle two neptunium ions depends on the ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s and solvents used.) The stability sequence for Np(IV), Np(V), and Np(VI) complexes with monovalent inorganic ligands is fluoride, F− > dihydrogen phosphate, > thiocyanate, SCN− > nitrate, > chloride, Cl− > perchlorate, ; the order for divalent inorganic ligands is carbonate, > Monohydrogen phosphate, > sulfate, . These follow the strengths of the corresponding acids. The divalent ligands are more strongly complexing than the monovalent ones. can also form the complex ions [] (M = Al, gallium, Ga, scandium, Sc, indium, In, iron, Fe, chromium, Cr, rhodium, Rh) in perchloric acid solution: the strength of interaction between the two cations follows the order Fe > In > Sc > Ga > Al. The neptunyl and uranyl ions can also form a complex together.
Applications
Precursor in plutonium-238 production
An important use of 237Np is as a precursor inplutonium-238
Plutonium-238 ( or Pu-238) is a radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suitable for usage ...
production, where it is irradiated with neutrons to form Plutonium-238, 238Pu, an alpha emitter for radioisotope thermal generator
A radioisotope thermoelectric generator (RTG, RITEG), or radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the Decay heat, heat released by the decay of a suitable radioactive material i ...
s for spacecraft and military applications. 237Np will capture a neutron to form 238Np and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
with a half-life of just over two days to 238Pu.
:spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
but would have to be separated from other isotopes of plutonium.Yoshida et al., pp. 702–3. Irradiating neptunium-237 with electron beams, provoking bremsstrahlung, also produces quite pure samples of the isotope plutonium-236, useful as a tracer to determine plutonium concentration in the environment.
Nuclear weapons
Neptunium is fissionable, and could theoretically be used as fuel in a fast-neutron reactor or a nuclear weapon, with acritical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
of around 60 kilograms. In 1992, the U.S. Department of Energy declassified the statement that neptunium-237 "can be used for a nuclear explosive device"."Restricted Data Declassification Decisions from 1946 until Present"accessed Sept 23, 2006. It is not believed that an actual weapon has ever been constructed using neptunium. As of 2009, the world production of neptunium-237 by commercial power reactors was over 1000 critical masses a year, but to extract the isotope from irradiated fuel elements would be a major industrial undertaking. In September 2002, researchers at the Los Alamos National Laboratory briefly produced the first known nuclear
critical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
using a significant fraction of neptunium, in combination with shells of enriched uranium (uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
), discovering that the critical mass of a bare sphere of neptunium-237 "ranges from kilogram weights in the high fifties to low sixties," showing that it "is about as good a bomb material as [uranium-235]." The United States Federal government made plans in March 2004 to move America's supply of separated neptunium to a nuclear-waste disposal site in Nevada.
Physics
237Np is used in devices for detecting high-energy (MeV) neutrons.Role in nuclear waste
Neptunium accumulates in commercial household ionization-chamber smoke detectors from decay of the (typically) 0.2 microgram of americium-241 initially present as a source of ionizing radiation. With a half-life of 432 years, the americium-241 in an smoke detector#Design, ionization smoke detector includes about 3% neptunium after 20 years, and about 15% after 100 years. Under Redox, oxidizing conditions, neptunium-237 is the most mobileactinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
in the deep geological repository environment of the Yucca Mountain project in Nevada. This makes it and its predecessors such as americium-241 candidates of interest for destruction by nuclear transmutation. Due to its long half-life, neptunium will become the major contributor of the total radiotoxicity at Yucca Mountain in 10,000 years. As it is unclear what happens to the non-reprocessed spent fuel containment in that long time span, an extraction and transmutation of neptunium after spent fuel reprocessing could help to minimize the contamination of the environment if the nuclear waste could be mobilized after several thousand years.
Biological role and precautions
Neptunium does not have a biological role, as it has a short half-life and occurs only in small traces naturally. Animal tests show it to be absorbed poorly (~1%) via the digestive tract. When injected it concentrates in the bones, from which it is slowly released. Finely divided neptunium metal presents a fire hazard because neptunium ispyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
; small grains will ignite spontaneously in air at room temperature.
References
Bibliography
* * * * * *Literature
* ''Guide to the Elements – Revised Edition'', Albert Stwertka, (Oxford University Press; 1998) * Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer-Verlag, Dordrecht 2006, . * * Eric Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011, .External links
Neptunium
at ''The Periodic Table of Videos'' (University of Nottingham)
Lab builds world's first neptunium sphere
, U.S. Department of Energy Research News
NLM Hazardous Substances Databank – Neptunium, Radioactive
Neptunium: Human Health Fact Sheet
{{Authority control Neptunium, Chemical elements Actinides Synthetic elements Nuclear materials Pyrophoric materials