Molecular Orbital Diagram on:
[Wikipedia]
[Google]
[Amazon]
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining
 With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. The σ from the 2p is more non-bonding due to mixing, and same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital.
The bond order of diatomic nitrogen is three, and it is a diamagnetic molecule.
The bond order for
With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. The σ from the 2p is more non-bonding due to mixing, and same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital.
The bond order of diatomic nitrogen is three, and it is a diamagnetic molecule.
The bond order for
 Oxygen has a similar setup to H2, but now we consider 2s and 2p orbitals. When creating the molecular orbitals from the p orbitals, the three atomic orbitals split into three molecular orbitals, a singly degenerate σ and a doubly degenerate π orbital. Another property we can observe by examining molecular orbital diagrams is the magnetic property of
Oxygen has a similar setup to H2, but now we consider 2s and 2p orbitals. When creating the molecular orbitals from the p orbitals, the three atomic orbitals split into three molecular orbitals, a singly degenerate σ and a doubly degenerate π orbital. Another property we can observe by examining molecular orbital diagrams is the magnetic property of
 Dimolybdenum ( Mo2) is notable for having a
Dimolybdenum ( Mo2) is notable for having a
 Nitric oxide is a heteronuclear molecule that exhibits mixing. The construction of its MO diagram is the same as for the homonuclear molecules. It has a bond order of 2.5 and is a paramagnetic molecule. The energy differences of the 2s orbitals are different enough that each produces its own non-bonding σ orbitals. Notice this is a good example of making the ionized NO+ stabilize the bond and generate a triple bond, also changing the magnetic property to diamagnetic.
Nitric oxide is a heteronuclear molecule that exhibits mixing. The construction of its MO diagram is the same as for the homonuclear molecules. It has a bond order of 2.5 and is a paramagnetic molecule. The energy differences of the 2s orbitals are different enough that each produces its own non-bonding σ orbitals. Notice this is a good example of making the ionized NO+ stabilize the bond and generate a triple bond, also changing the magnetic property to diamagnetic.

Image:Atomic Orbitals CO2.svg, Atomic orbitals of carbon dioxide
Image:Molecular Orbitals CO2.svg, Molecular orbitals of carbon dioxide
Image:MO Diagram CO2.svg, MO diagram of carbon dioxide
 The oxygen atomic orbitals are labeled according to their symmetry as a1 for the 2s orbital and b1 (2px), b2 (2py) and a1 (2pz) for the three 2p orbitals. The two hydrogen 1s orbitals are premixed to form a1 (σ) and b2 (σ*) MO.
Mixing takes place between same-symmetry orbitals of comparable energy resulting a new set of MO's for water:
* 2a1 MO from mixing of the oxygen 2s AO and the hydrogen σ MO.
* 1b2 MO from mixing of the oxygen 2py AO and the hydrogen σ* MO.
* 3a1 MO from mixing of the a1 AOs.
* 1b1 nonbonding MO from the oxygen 2px AO (the p-orbital perpendicular to the molecular plane).
In agreement with this description the photoelectron spectrum for water shows a sharp peak for the nonbonding 1b1 MO (12.6 eV) and three broad peaks for the 3a1 MO (14.7 eV), 1b2 MO (18.5 eV) and the 2a1 MO (32.2 eV). The 1b1 MO is a lone pair, while the 3a1, 1b2 and 2a1 MO's can be localized to give two O−H bonds and an in-plane lone pair. This MO treatment of water does not have two equivalent ''rabbit ear'' lone pairs.
The oxygen atomic orbitals are labeled according to their symmetry as a1 for the 2s orbital and b1 (2px), b2 (2py) and a1 (2pz) for the three 2p orbitals. The two hydrogen 1s orbitals are premixed to form a1 (σ) and b2 (σ*) MO.
Mixing takes place between same-symmetry orbitals of comparable energy resulting a new set of MO's for water:
* 2a1 MO from mixing of the oxygen 2s AO and the hydrogen σ MO.
* 1b2 MO from mixing of the oxygen 2py AO and the hydrogen σ* MO.
* 3a1 MO from mixing of the a1 AOs.
* 1b1 nonbonding MO from the oxygen 2px AO (the p-orbital perpendicular to the molecular plane).
In agreement with this description the photoelectron spectrum for water shows a sharp peak for the nonbonding 1b1 MO (12.6 eV) and three broad peaks for the 3a1 MO (14.7 eV), 1b2 MO (18.5 eV) and the 2a1 MO (32.2 eV). The 1b1 MO is a lone pair, while the 3a1, 1b2 and 2a1 MO's can be localized to give two O−H bonds and an in-plane lone pair. This MO treatment of water does not have two equivalent ''rabbit ear'' lone pairs.
Link
*MO diagrams at chem1.co
*Molecular orbitals at winter.group.shef.ac.u
{{Chemical bonding theory Chemical bonding
chemical bonding
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in ...
in molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s in terms of molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, whic ...
in general and the linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
(LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s combine to form the same number of molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s, although the electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
s such as dihydrogen
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatom ...
, dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (). Others are:
* Ato ...
, and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transition
In atomic physics and chemistry, an atomic electron transition (also called an atomic transition, quantum jump, or quantum leap) is an electron changing from one energy level to another within an atom or artificial atom. The time scale of a qua ...
s that can take place.
History
Qualitative MO theory was introduced in 1928 by Robert S. Mulliken andFriedrich Hund
Friedrich Hermann Hund (4 February 1896 – 31 March 1997) was a German physicist from Karlsruhe known for his work on atoms and molecules. He is known for the Hund's rules to predict the electron configuration of chemical elements. His work on H ...
. A mathematical description was provided by contributions from Douglas Hartree
Douglas Rayner Hartree (27 March 1897 – 12 February 1958) was an English mathematician and physicist most famous for the development of numerical analysis and its application to the Hartree–Fock equations of atomic physics and the c ...
in 1928 and Vladimir Fock in 1930.
Basics
Molecular orbital diagrams are diagrams of molecular orbital (MO)energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Degenerate energy levels are commonly shown side by side. Appropriate AO and MO levels are filled with electrons by the Pauli Exclusion Principle, symbolized by small vertical arrows whose directions indicate the electron spin
Spin is an intrinsic form of angular momentum carried by elementary particles, and thus by composite particles such as hadrons, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic ...
s. The AO or MO shapes themselves are often not shown on these diagrams. For a diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. For simple polyatomic molecules with a "central atom" such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
() or carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(), a MO diagram may show one of the identical bonds to the central atom. For other polyatomic molecules, an MO diagram may show one or more bonds of interest in the molecules, leaving others out for simplicity. Often even for simple molecules, AO and MO levels of inner orbitals and their electrons may be omitted from a diagram for simplicity.
In MO theory molecular orbitals form by the overlap of atomic orbitals. Because σ bonds feature greater overlap than π bonds, σ bonding and σ* antibonding
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more node ...
orbitals feature greater energy splitting (separation) than π and π* orbitals. The atomic orbital energy correlates with electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
as more electronegative atoms hold their electrons more tightly, lowering their energies. Sharing of molecular orbitals between atoms is more important when the atomic orbitals have comparable energy; when the energies differ greatly the orbitals tend to be localized on one atom and the mode of bonding becomes ionic. A second condition for overlapping atomic orbitals is that they have the same symmetry.
Two atomic orbitals can overlap in two ways depending on their phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
*Phase space, a mathematica ...
relationship (or relative signs for real orbitals). The phase (or sign) of an orbital is a direct consequence of the wave-like properties of electrons. In graphical representations of orbitals, orbital phase is depicted either by a plus or minus sign (which has no relationship to electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
) or by shading one lobe. The sign of the phase itself does not have physical meaning except when mixing orbitals to form molecular orbitals.
Two same-sign orbitals have a constructive overlap forming a molecular orbital with the bulk of the electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
located between the two nuclei. This MO is called the bonding orbital and its energy is lower than that of the original atomic orbitals. A bond involving molecular orbitals which are symmetric with respect to any rotation around the bond axis is called a sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
(σ-bond). If the phase cycles once while rotating round the axis, the bond is a pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
(π-bond). Symmetry labels are further defined by whether the orbital maintains its original character after an inversion about its center; if it does, it is defined gerade
In physics, a parity transformation (also called parity inversion) is the flip in the sign of ''one'' spatial coordinate. In three dimensions, it can also refer to the simultaneous flip in the sign of all three spatial coordinates (a point ref ...
, ''g''. If the orbital does not maintain its original character, it is ungerade, ''u''.
Atomic orbitals can also interact with each other out-of-phase which leads to destructive cancellation and no electron density between the two nuclei at the so-called nodal plane depicted as a perpendicular dashed line. In this anti-bonding MO with energy much higher than the original AO's, any electrons present are located in lobes pointing away from the central internuclear axis. For a corresponding σ-bonding orbital, such an orbital would be symmetrical but differentiated from it by an asterisk
The asterisk ( ), from Late Latin , from Ancient Greek , , "little star", is a Typography, typographical symbol. It is so called because it resembles a conventional image of a star (heraldry), heraldic star.
Computer scientists and Mathematici ...
as in σ*. For a π-bond, corresponding bonding and antibonding orbitals would not have such symmetry around the bond axis and be designated π and π*, respectively.
The next step in constructing an MO diagram is filling the newly formed molecular orbitals with electrons. Three general rules apply:
*The Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
states that orbitals are filled starting with the lowest energy
*The Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle (German: Pauli-Ausschlussprinzip) states that two or more identical particles with half-integer spins (i.e. fermions) cannot simultaneously occupy the same quantum state within a system that o ...
states that the maximum number of electrons occupying an orbital is two, with opposite spins
* Hund's rule states that when there are several MO's with equal energy, the electrons occupy the MO's one at a time before two electrons occupy the same MO.
The filled MO highest in energy is called the highest occupied molecular orbital (HOMO) and the empty MO just above it is then the lowest unoccupied molecular orbital (LUMO). The electrons in the bonding MO's are called bonding electrons and any electrons in the antibonding orbital would be called antibonding electrons. The reduction in energy of these electrons is the driving force for chemical bond formation. Whenever mixing for an atomic orbital is not possible for reasons of symmetry or energy, a non-bonding MO is created, which is often quite similar to and has energy level equal or close to its constituent AO, thus not contributing to bonding energetics. The resulting electron configuration can be described in terms of bond type, parity and occupancy for example dihydrogen 1σ''g''2. Alternatively it can be written as a molecular term symbol e.g. 1 Σg+ for dihydrogen. Sometimes, the letter n is used to designate a non-bonding orbital.
For a stable bond, the bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
defined as
must be positive.
The relative order in MO energies and occupancy corresponds with electronic transitions found in photoelectron spectroscopy (PES). In this way it is possible to experimentally verify MO theory. In general, sharp PES transitions indicate nonbonding electrons and broad bands are indicative of bonding and antibonding delocalized electrons. Bands can resolve into fine structure with spacings corresponding to vibrational modes of the molecular cation (see Franck–Condon principle). PES energies are different from ionisation energies which relates to the energy required to strip off the th electron after the first electrons have been removed. MO diagrams with energy values can be obtained mathematically using the Hartree–Fock method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The method is named after Douglas ...
. The starting point for any MO diagram is a predefined molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
for the molecule in question. An exact relationship between geometry and orbital energies is given in Walsh diagrams.
s-p mixing
The phenomenon of s-p mixing occurs when molecular orbitals of the same symmetry formed from the combination of 2s and 2p atomic orbitals are close enough in energy to further interact, which can lead to a change in the expected order of orbital energies. When molecular orbitals are formed, they are mathematically obtained from linear combinations of the starting atomic orbitals. Generally, in order to predict their relative energies, it is sufficient to consider only one atomic orbital from each atom to form a pair of molecular orbitals, as the contributions from the others are negligible. For instance, in dioxygen the 3σg MO can be roughly considered to be formed from interaction of oxygen 2pz AOs only. It is found to be lower in energy than the 1πu MO, both experimentally and from more sophisticated computational models, so that the expected order of filling is the 3σg before the 1πu. Hence the approximation to ignore the effects of further interactions is valid. However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO show that the 3σg MO is higher in energy than (and therefore filled after) the 1πu MO. This can be rationalised as the first-approximation 3σg has a suitable symmetry to interact with the 2σg bonding MO formed from the 2s AOs. As a result, the 2σg is lowered in energy, whilst the 3σg is raised. For the aforementioned molecules this results in the 3σg being higher in energy than the 1πu MO, which is where s-p mixing is most evident. Likewise, interaction between the 2σu* and 3σu* MOs leads to a lowering in energy of the former and a raising in energy of the latter. However this is of less significance than the interaction of the bonding MOs.Diatomic MO diagrams
A diatomic molecular orbital diagram is used to understand the bonding of adiatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
. MO diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
. They also give insight to the bond order of the molecule, how many bonds are shared between the two atoms.
The energies of the electrons are further understood by applying the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
to a molecule. Quantum Mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
is able to describe the energies exactly for single electron systems but can be approximated precisely for multiple electron systems using the Born-Oppenheimer Approximation, such that the nuclei are assumed stationary. The LCAO-MO method is used in conjunction to further describe the state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
of the molecule.
Diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
s consist of a bond between only two atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. They can be broken into two categories: homonuclear and heteronuclear. A homonuclear diatomic molecule is one composed of two atoms of the same element. Examples are H2, O2, and N2. A heteronuclear diatomic molecule is composed of two atoms of two different elements. Examples include CO, HCl, and NO.
Dihydrogen
The smallest molecule,hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas exists as dihydrogen (H-H) with a single covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
for its electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, the bond forms by overlap of these two atomic orbitals. In the figure the two atomic orbitals are depicted on the left and on the right. The vertical axis always represents the orbital energies. Each atomic orbital is singly occupied with an up or down arrow representing an electron.
Application of MO theory for dihydrogen results in having both electrons in the bonding MO with electron configuration 1σ''g''2. The bond order for dihydrogen is (2-0)/2 = 1. The photoelectron spectrum of dihydrogen shows a single set of multiplets between 16 and 18 eV (electron volts).
The dihydrogen MO diagram helps explain how a bond breaks. When applying energy to dihydrogen, a molecular electronic transition
In theoretical chemistry, molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy level. The energy change associated with this transition provides information on the structur ...
takes place when one electron in the bonding MO is promoted to the antibonding MO. The result is that there is no longer a net gain in energy.
The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ* molecular orbitals. Two atomic orbitals in phase create a larger electron density, which leads to the σ orbital. If the two 1s orbitals are not in phase, a node between them causes a jump in energy, the σ* orbital. From the diagram you can deduce the bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
, how many bonds are formed between the two atoms. For this molecule it is equal to one. Bond order can also give insight to how close or stretched a bond has become if a molecule is ionized.
Dihelium and diberyllium
Dihelium (He-He) is a hypothetical molecule and MO theory helps to explain why dihelium does not exist in nature. The MO diagram for dihelium looks very similar to that of dihydrogen, but each helium has two electrons in its 1s atomic orbital rather than one for hydrogen, so there are now four electrons to place in the newly formed molecular orbitals. The only way to accomplish this is by occupying both the bonding and antibonding orbitals with two electrons, which reduces the bond order ((2−2)/2) to zero and cancels the net energy stabilization. However, by removing one electron from dihelium, the stable gas-phase species ion is formed with bond order 1/2. Another molecule that is precluded based on this principle is diberyllium.Beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
has an electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
1s22s2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. This mixing makes the antibonding 1σu orbital slightly less antibonding than the bonding 1σg orbital is bonding, with a net effect that the whole configuration has a slight bonding nature. This explains the fact that the diberyllium molecule exists and has been observed in the gas phase. The slight bonding nature explains the low dissociation energy of only 59 kJ·mol−1.
Dilithium
MO theory correctly predicts that dilithium is a stable molecule with bond order 1 (configuration 1σ''g''21σ''u''22σ''g''2). The 1s MOs are completely filled and do not participate in bonding. Dilithium is a gas-phase molecule with a much lowerbond strength
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bo ...
than dihydrogen because the 2s electrons are further removed from the nucleus. In a more detailed analysis which considers the environment of each orbital due to all other electrons, both the 1σ orbitals have higher energies than the 1s AO and the occupied 2σ is also higher in energy than the 2s AO (see table 1).
Diboron
The MO diagram for diboron (B-B, electron configuration 1σ''g''21σ''u''22σ''g''22σ''u''21π''u''2) requires the introduction of an atomic orbital overlap model for p orbitals. The threedumbbell
The dumbbell, a type of free weight, is a piece of equipment used in weight training. It is usually used individually and/or in pairs, with one in each hand.
History
The forerunner of the dumbbell, halteres, were used in ancient Greece as li ...
-shaped p-orbitals have equal energy and are oriented mutually perpendicularly (or orthogonal
In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic ...
ly). The p-orbitals oriented in the z-direction (pz) can overlap end-on forming a bonding (symmetrical) σ orbital and an antibonding σ* molecular orbital. In contrast to the sigma 1s MO's, the σ 2p has some non-bonding electron density at either side of the nuclei and the σ* 2p has some electron density between the nuclei.
The other two p-orbitals, py and px, can overlap side-on. The resulting bonding orbital has its electron density in the shape of two lobes above and below the plane of the molecule. The orbital is not symmetric around the molecular axis and is therefore a pi orbital
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occ ...
. The antibonding pi orbital (also asymmetrical) has four lobes pointing away from the nuclei. Both py and px orbitals form a pair of pi orbitals equal in energy ( degenerate) and can have higher or lower energies than that of the sigma orbital.
In diboron the 1s and 2s electrons do not participate in bonding but the single electrons in the 2p orbitals occupy the 2πpy and the 2πpx MO's resulting in bond order 1. Because the electrons have equal energy (they are degenerate) diboron is a diradical
In chemistry, a diradical is a chemical species, molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate energy level, degenerate. The term "diradical" is mainly used to describe organic compounds, where most ...
and since the spins are parallel the molecule is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
.
In certain diborynes the boron atoms are excited and the bond order is 3.
Dicarbon
Like diboron, dicarbon (C-Celectron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
:1σg21σu22σg22σu21πu4) is a reactive gas-phase molecule. The molecule can be described as having two pi bonds but without a sigma bond.
Dinitrogen
 With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. The σ from the 2p is more non-bonding due to mixing, and same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital.
The bond order of diatomic nitrogen is three, and it is a diamagnetic molecule.
The bond order for
With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. The σ from the 2p is more non-bonding due to mixing, and same with the 2s σ. This also causes a large jump in energy in the 2p σ* orbital.
The bond order of diatomic nitrogen is three, and it is a diamagnetic molecule.
The bond order for dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
(1σg21σu22σg22σu21πu43σg2) is three because two electrons are now also added in the 3σ MO. The MO diagram correlates with the experimental photoelectron spectrum for nitrogen. The 1σ electrons can be matched to a peak at 410 eV (broad), the 2σg electrons at 37 eV (broad), the 2σu electrons at 19 eV (doublet), the 1πu4 electrons at 17 eV (multiplets), and finally the 3σg2 at 15.5 eV (sharp).
Dioxygen
 Oxygen has a similar setup to H2, but now we consider 2s and 2p orbitals. When creating the molecular orbitals from the p orbitals, the three atomic orbitals split into three molecular orbitals, a singly degenerate σ and a doubly degenerate π orbital. Another property we can observe by examining molecular orbital diagrams is the magnetic property of
Oxygen has a similar setup to H2, but now we consider 2s and 2p orbitals. When creating the molecular orbitals from the p orbitals, the three atomic orbitals split into three molecular orbitals, a singly degenerate σ and a doubly degenerate π orbital. Another property we can observe by examining molecular orbital diagrams is the magnetic property of diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
or paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. If all the electrons are paired, there is a slight repulsion and it is classified as diamagnetic. If unpaired electrons are present, it is attracted to a magnetic field, and therefore paramagnetic. Oxygen is an example of a paramagnetic diatomic. The bond order of diatomic oxygen is two.
MO treatment of dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (). Others are:
* Ato ...
is different from that of the previous diatomic molecules because the pσ MO is now lower in energy than the 2π orbitals. This is attributed to interaction between the 2s MO and the 2pz MO.''Modern Inorganic Chemistry'' William L. Jolly (McGraw-Hill 1984), p.106 Distributing 8 electrons over 6 molecular orbitals leaves the final two electrons as a degenerate pair in the 2pπ* antibonding orbitals resulting in a bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
of 2. As in diboron, these two unpaired electrons have the same spin in the ground state, which is a paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
diradical
In chemistry, a diradical is a chemical species, molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate energy level, degenerate. The term "diradical" is mainly used to describe organic compounds, where most ...
triplet oxygen
Triplet oxygen, 3O2, refers to the ''S'' = 1 electronic ground state of molecular oxygen (dioxygen). Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unusual example of a stable and commonly encountered diradi ...
. The first excited state has both HOMO electrons paired in one orbital with opposite spins, and is known as singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemistry, inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are Radical (chemistry), spin p ...
.
The bond order decreases and the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
increases in the order (112.2 pm), (121 pm), (128 pm) and (149 pm).
Difluorine and dineon
In difluorine two additional electrons occupy the 2pπ* with a bond order of 1. In dineon (as with dihelium) the number of bonding electrons equals the number of antibonding electrons and this molecule does not exist.Dimolybdenum and ditungsten
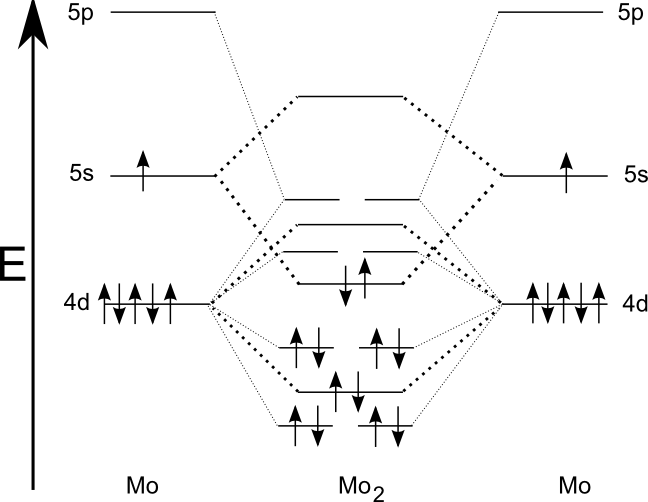
 Dimolybdenum ( Mo2) is notable for having a
Dimolybdenum ( Mo2) is notable for having a sextuple bond
A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple bonds are the diatomic dimolybdenum ( Mo2) and ditungsten ( W2), which exist in the gaseous ph ...
. This involves two sigma bonds (4dz2 and 5s), two pi bonds (using 4dxz and 4dyz), and two delta bond
In chemistry, a delta bond (δ bond) is a Covalent bond, covalent chemical bond, in which four lobes of an atomic orbital on one atom orbital overlap, overlap four lobes of an atomic orbital on another atom. This overlap leads to the formation o ...
s (4dx2 − y2 and 4dxy). Ditungsten ( W2) has a similar structure.
MO energies overview
Table 1 gives an overview of MO energies for first row diatomic molecules calculated by the Hartree-Fock-Roothaan method, together with atomic orbital energies.Heteronuclear diatomics
In heteronuclear diatomic molecules, mixing of atomic orbitals only occurs when theelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
values are similar. In carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO, isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with dinitrogen) the oxygen 2s orbital is much lower in energy than the carbon 2s orbital and therefore the degree of mixing is low. The electron configuration 1σ21σ*22σ22σ*21π43σ2 is identical to that of nitrogen. The g and u subscripts no longer apply because the molecule lacks a center of symmetry.
In hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
(HF), the hydrogen 1s orbital can mix with fluorine 2pz orbital to form a sigma bond because experimentally the energy of 1s of hydrogen is comparable with 2p of fluorine. The HF electron configuration 1σ22σ23σ21π4 reflects that the other electrons remain in three lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s and that the bond order is 1.
The more electronegative atom is the more energetically excited because it more similar in energy to its atomic orbital. This also accounts for the majority of the electron negativity residing around the more electronegative molecule. Applying the LCAO-MO method allows us to move away from a more static Lewis structure type approach and actually account for periodic trends that influence electron movement. Non-bonding orbitals refer to lone pairs seen on certain atoms in a molecule. A further understanding for the energy level refinement can be acquired by delving into quantum chemistry; the Schrödinger equation can be applied to predict movement and describe the state of the electrons in a molecule.
NO
 Nitric oxide is a heteronuclear molecule that exhibits mixing. The construction of its MO diagram is the same as for the homonuclear molecules. It has a bond order of 2.5 and is a paramagnetic molecule. The energy differences of the 2s orbitals are different enough that each produces its own non-bonding σ orbitals. Notice this is a good example of making the ionized NO+ stabilize the bond and generate a triple bond, also changing the magnetic property to diamagnetic.
Nitric oxide is a heteronuclear molecule that exhibits mixing. The construction of its MO diagram is the same as for the homonuclear molecules. It has a bond order of 2.5 and is a paramagnetic molecule. The energy differences of the 2s orbitals are different enough that each produces its own non-bonding σ orbitals. Notice this is a good example of making the ionized NO+ stabilize the bond and generate a triple bond, also changing the magnetic property to diamagnetic.
HF

Hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
is another example of a heteronuclear molecule. It is slightly different in that the π orbital is non-bonding, as well as the 2s σ. From the hydrogen, its valence 1s electron interacts with the 2p electrons of fluorine. This molecule is diamagnetic and has a bond order of one.
Triatomic molecules
Carbon dioxide
Carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, , is a linear molecule with a total of sixteen bonding electrons in its valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
. Carbon is the central atom of the molecule and a principal axis, the z-axis, is visualized as a single axis that goes through the center of carbon and the two oxygens atoms.
For convention, blue atomic orbital lobes are positive phases, red atomic orbitals are negative phases, with respect to the wave function from the solution of the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
. In carbon dioxide the carbon 2s (−19.4 eV), carbon 2p (−10.7 eV), and oxygen 2p (−15.9 eV)) energies associated with the atomic orbitals are in proximity whereas the oxygen 2s energy (−32.4 eV) is different.
Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into px, py, and pz. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
(''π'') or generates no phase change, known as a sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
(''σ''). Symmetry labels are further defined by whether the atomic orbital maintains its original character after an inversion about its center atom; if the atomic orbital does retain its original character it is defined gerade
In physics, a parity transformation (also called parity inversion) is the flip in the sign of ''one'' spatial coordinate. In three dimensions, it can also refer to the simultaneous flip in the sign of all three spatial coordinates (a point ref ...
, ''g'', or if the atomic orbital does not maintain its original character, ungerade, ''u''. The final symmetry-labeled atomic orbital is now known as an irreducible representation.
Carbon dioxide’s molecular orbitals are made by the linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
of the same irreducible representation that are also similar in atomic orbital energy. Significant atomic orbital overlap explains why sp bonding may occur. Strong mixing of the oxygen 2s atomic orbital is not to be expected and are non-bonding degenerate molecular orbitals. The combination of similar atomic orbital/wave functions and the combinations of atomic orbital/wave function inverses create particular energies associated with the nonbonding (no change), bonding (lower than either parent orbital energy) and antibonding
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more node ...
(higher energy than either parent atomic orbital energy) molecular orbitals.
Water
For nonlinear molecules, the orbital symmetries are not σ or π but depend on the symmetry of each molecule. Water () is a bent molecule (105°) with C2vmolecular symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
. The possible orbital symmetries are listed in the table below. For example, an orbital of B1 symmetry (called a b1 orbital with a small b since it is a one-electron function) is multiplied by -1 under the symmetry operations C2 (rotation about the 2-fold rotation axis) and σv'(yz) (reflection in the molecular plane). It is multiplied by +1(unchanged) by the identity operation E and by σv(xz) (reflection in the plane bisecting the H-O-H angle).
Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(H2S) too has a C2v symmetry with 8 valence electrons but the bending angle is only 92°. As reflected in its photoelectron spectrum as compared to water the 5a1 MO (corresponding to the 3a1 MO in water) is stabilised (improved overlap) and the 2b2 MO (corresponding to the 1b2 MO in water) is destabilized (poorer overlap).
References
External links
*MO diagrams at meta-synthesis.coLink
*MO diagrams at chem1.co
*Molecular orbitals at winter.group.shef.ac.u
{{Chemical bonding theory Chemical bonding