Methane Hydrates on:
[Wikipedia]
[Google]
[Amazon]
Methane clathrate (CH4·5.75H2O) or (4CH4·23H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a
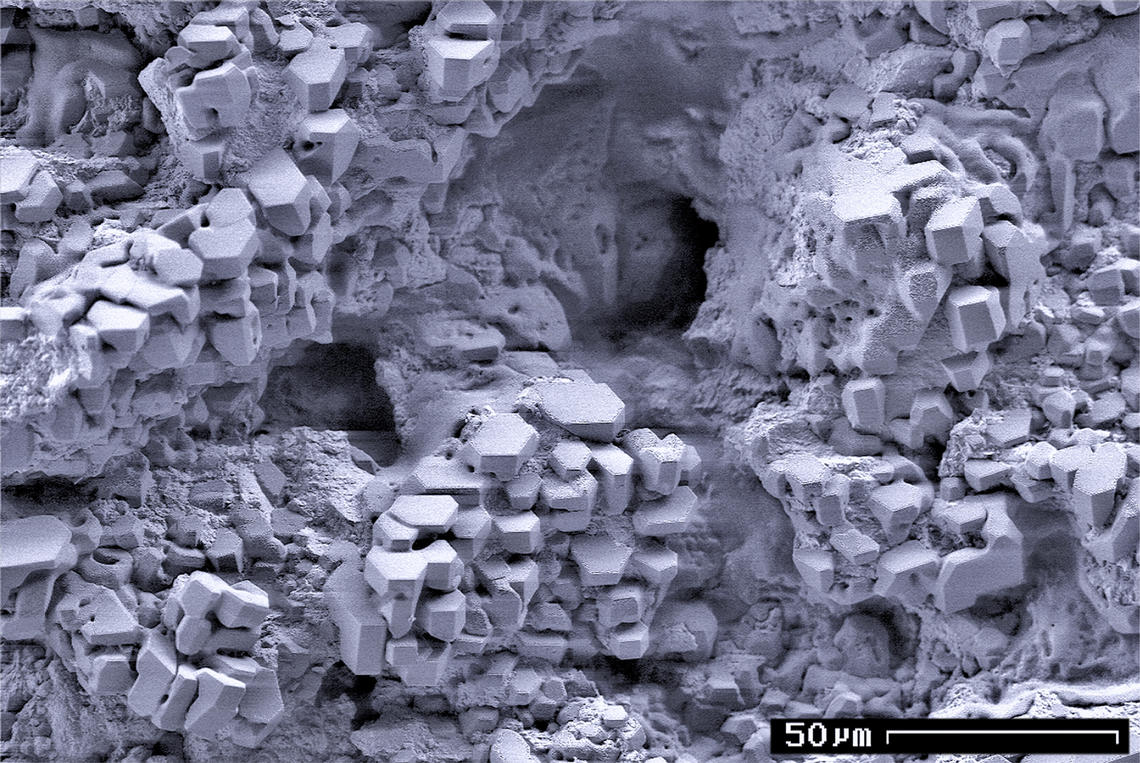 The nominal methane clathrate hydrate composition is (CH4)4(H2O)23, or 1 mole of methane for every 5.75 moles of water, corresponding to 13.4% methane by mass, although the actual composition is dependent on how many methane molecules fit into the various cage structures of the water lattice. The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment. One litre of fully saturated methane clathrate solid would therefore contain about 120 grams of methane (or around 169 litres of methane gas at 0 °C and 1 atm), or one cubic metre of methane clathrate releases about 160 cubic metres of gas.
Methane forms a "structure-I" hydrate with two dodecahedral (12 vertices, thus 12 water molecules) and six tetradecahedral (14 water molecules) water cages per unit cell. (Because of sharing of water molecules between cages, there are only 46 water molecules per unit cell.) This compares with a hydration number of 20 for methane in aqueous solution. Note: the number 20 is called a magic number equal to the number found for the amount of water molecules surrounding a
The nominal methane clathrate hydrate composition is (CH4)4(H2O)23, or 1 mole of methane for every 5.75 moles of water, corresponding to 13.4% methane by mass, although the actual composition is dependent on how many methane molecules fit into the various cage structures of the water lattice. The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment. One litre of fully saturated methane clathrate solid would therefore contain about 120 grams of methane (or around 169 litres of methane gas at 0 °C and 1 atm), or one cubic metre of methane clathrate releases about 160 cubic metres of gas.
Methane forms a "structure-I" hydrate with two dodecahedral (12 vertices, thus 12 water molecules) and six tetradecahedral (14 water molecules) water cages per unit cell. (Because of sharing of water molecules between cages, there are only 46 water molecules per unit cell.) This compares with a hydration number of 20 for methane in aqueous solution. Note: the number 20 is called a magic number equal to the number found for the amount of water molecules surrounding a 
 The size of the oceanic methane clathrate reservoir is poorly known, and estimates of its size decreased by roughly an
The size of the oceanic methane clathrate reservoir is poorly known, and estimates of its size decreased by roughly an
 At sufficient depths, methane complexes directly with water to form methane hydrates, as was observed during the
At sufficient depths, methane complexes directly with water to form methane hydrates, as was observed during the
Are there deposits of methane under the sea? Will global warming release the methane to the atmosphere?
(2007)
Methane seeps from Arctic sea bed
(BBC)
Bubbles of warming, beneath the ice
(LA Times 2009)
online calculator : hydrate formation conditions with different EOSs
Centre for Arctic Gas Hydrate, Environment and Climate (CAGE)Center for Hydrate Research
* ttps://web.archive.org/web/20070105164147/http://www.eee.columbia.edu/research-projects/sustainable_energy/Hydrates/index.html Carbon Neutral Methane Energy Production from Hydrate Deposits(Columbia University)
USGS Gas Hydrates Lab
(2012)
Ancient Methane Explosions Created Ocean Craters
(2017) {{DEFAULTSORT:Methane Clathrate Clathrate hydrates Hydrocarbons Methane Unconventional gas Natural gas
clathrate hydrate
Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
) in which a large amount of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
is trapped within a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
structure of water, forming a solid similar to ice
Ice is water that is frozen into a solid state, typically forming at or below temperatures of 0 ° C, 32 ° F, or 273.15 K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally oc ...
. Originally thought to occur only in the outer regions of the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
s on the ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
floors of the Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
(around 1100m below the sea level). Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans.
Methane clathrates are common constituents of the shallow marine geosphere
There are several conflicting usages of geosphere, variously defined.
In Aristotelian physics, the term was applied to four spherical ''natural places'', concentrically nested around the center of the Earth, as described in the lectures '' Ph ...
and they occur in deep sedimentary
Sedimentary rocks are types of rock formed by the cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or deposited at Earth's surface. Sedime ...
structures and form outcrop
An outcrop or rocky outcrop is a visible exposure of bedrock or ancient superficial deposits on the surface of the Earth and other terrestrial planets.
Features
Outcrops do not cover the majority of the Earth's land surface because in most p ...
s on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from deep along geological faults. Precipitation occurs when the methane comes in contact with water within the sea bed subject to temperature and pressure. In 2008, research on Antarctic Vostok Station and EPICA Dome C ice cores revealed that methane clathrates were also present in deep Antarctic
The Antarctic (, ; commonly ) is the polar regions of Earth, polar region of Earth that surrounds the South Pole, lying within the Antarctic Circle. It is antipodes, diametrically opposite of the Arctic region around the North Pole.
The Antar ...
ice core
An ice core is a core sample that is typically removed from an ice sheet or a high mountain glacier
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier ...
s and record a history of atmospheric methane
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change. Methane is one of the most potent greenhouse gases. Methane's radiati ...
concentrations, dating to 800,000 years ago. The ice-core methane clathrate record is a primary source of data for global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
research, along with oxygen and carbon dioxide.
Methane clathrates used to be considered as a potential source of abrupt climate change, following the clathrate gun hypothesis
The clathrate gun hypothesis is a proposed explanation for the periods of rapid warming during the Quaternary. The hypothesis is that changes in fluxes in upper intermediate waters in the ocean caused temperature fluctuations that alternately accu ...
. In this scenario, heating causes catastrophic melting and breakdown of primarily undersea hydrates, leading to a massive release of methane and accelerating warming. Current research shows that hydrates react very slowly to warming, and that it's very difficult for methane to reach the atmosphere after dissociation. Some active seeps instead act as a minor carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
, because with the majority of methane dissolved underwater and encouraging methanotroph
Methanotrophs (sometimes called methanophiles) are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to ...
communities, the area around the seep also becomes more suitable for phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
. As the result, methane hydrates are no longer considered one of the tipping points in the climate system
In Climatology, climate science, a tipping point is a critical threshold that, when crossed, leads to large, accelerating and often irreversible changes in the climate system. If tipping points are crossed, they are likely to have severe impac ...
, and according to the IPCC Sixth Assessment Report
The Sixth Assessment Report (AR6) of the United Nations (UN) Intergovernmental Panel on Climate Change (IPCC) is the sixth in a series of reports which assess the available scientific information on climate change. Three Working Groups (WGI, II, ...
, no "detectable" impact on the global temperatures will occur in this century through this mechanism. Over several millennia, a more substantial response may still be seen.
General
Methane hydrates were discovered in Russia in the 1960s, and studies for extracting gas from it emerged at the beginning of the 21st century.Structure and composition
 The nominal methane clathrate hydrate composition is (CH4)4(H2O)23, or 1 mole of methane for every 5.75 moles of water, corresponding to 13.4% methane by mass, although the actual composition is dependent on how many methane molecules fit into the various cage structures of the water lattice. The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment. One litre of fully saturated methane clathrate solid would therefore contain about 120 grams of methane (or around 169 litres of methane gas at 0 °C and 1 atm), or one cubic metre of methane clathrate releases about 160 cubic metres of gas.
Methane forms a "structure-I" hydrate with two dodecahedral (12 vertices, thus 12 water molecules) and six tetradecahedral (14 water molecules) water cages per unit cell. (Because of sharing of water molecules between cages, there are only 46 water molecules per unit cell.) This compares with a hydration number of 20 for methane in aqueous solution. Note: the number 20 is called a magic number equal to the number found for the amount of water molecules surrounding a
The nominal methane clathrate hydrate composition is (CH4)4(H2O)23, or 1 mole of methane for every 5.75 moles of water, corresponding to 13.4% methane by mass, although the actual composition is dependent on how many methane molecules fit into the various cage structures of the water lattice. The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment. One litre of fully saturated methane clathrate solid would therefore contain about 120 grams of methane (or around 169 litres of methane gas at 0 °C and 1 atm), or one cubic metre of methane clathrate releases about 160 cubic metres of gas.
Methane forms a "structure-I" hydrate with two dodecahedral (12 vertices, thus 12 water molecules) and six tetradecahedral (14 water molecules) water cages per unit cell. (Because of sharing of water molecules between cages, there are only 46 water molecules per unit cell.) This compares with a hydration number of 20 for methane in aqueous solution. Note: the number 20 is called a magic number equal to the number found for the amount of water molecules surrounding a hydronium ion
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in ...
. A methane clathrate MAS NMR spectrum recorded at 275 K and 3.1 MPa
MPA or mPa may refer to:
Academia
Academic degrees
* Master of Performing Arts
* Master of Professional Accountancy
* Master of Public Administration
* Master of Public Affairs
Schools
* Mesa Preparatory Academy
* Morgan Park Academy
* M ...
shows a peak for each cage type and a separate peak for gas phase
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a ...
methane. In 2003, a clay-methane hydrate intercalate was synthesized in which a methane hydrate complex was introduced at the interlayer of a sodium-rich montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite grou ...
clay. The upper temperature stability of this phase is similar to that of structure-I hydrate.

Natural deposits
Methane clathrates are restricted to the shallowlithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
(i.e. < 2,000 m depth). Furthermore, necessary conditions are found only in either continental sedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
s in polar regions where average surface temperatures are less than 0 °C; or in oceanic sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
at water depths greater than 300 m where the bottom water temperature is around 2 °C. In addition, deep fresh water lakes may host gas hydrates as well, e.g. the fresh water Lake Baikal
Lake Baikal is a rift lake and the deepest lake in the world. It is situated in southern Siberia, Russia between the Federal subjects of Russia, federal subjects of Irkutsk Oblast, Irkutsk Oblasts of Russia, Oblast to the northwest and the Repu ...
, Siberia. Continental deposits have been located in Siberia
Siberia ( ; , ) is an extensive geographical region comprising all of North Asia, from the Ural Mountains in the west to the Pacific Ocean in the east. It has formed a part of the sovereign territory of Russia and its predecessor states ...
and Alaska
Alaska ( ) is a non-contiguous U.S. state on the northwest extremity of North America. Part of the Western United States region, it is one of the two non-contiguous U.S. states, alongside Hawaii. Alaska is also considered to be the north ...
in sandstone
Sandstone is a Clastic rock#Sedimentary clastic rocks, clastic sedimentary rock composed mainly of grain size, sand-sized (0.0625 to 2 mm) silicate mineral, silicate grains, Cementation (geology), cemented together by another mineral. Sand ...
and siltstone
Siltstone, also known as aleurolite, is a clastic sedimentary rock that is composed mostly of silt. It is a form of mudrock with a low clay mineral content, which can be distinguished from shale by its lack of fissility.
Although its permeabil ...
beds at less than 800 m depth. Oceanic deposits seem to be widespread in the continental shelf
A continental shelf is a portion of a continent that is submerged under an area of relatively shallow water, known as a shelf sea. Much of these shelves were exposed by drops in sea level during glacial periods. The shelf surrounding an islan ...
(see Fig.) and can occur within the sediments at depth or close to the sediment–water interface. They may cap even larger deposits of gaseous methane.
Oceanic
Methane hydrate can occur in various forms like massive, dispersed within pore spaces, nodules, veins/fractures/faults, and layered horizons. Generally, it is found unstable at standard pressure and temperature conditions, and 1 m3 of methane hydrate upon dissociation yields about 164 m3 of methane and 0.87 m3 of freshwater. There are two distinct types of oceanic deposits. The most common is dominated (> 99%) bymethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
contained in a structure I clathrate
A clathrate is a chemical substance consisting of a lattice (group), lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin language, Latin (), meaning 'with bars, Crystal structure, latticed'. Most clathrate ...
and generally found at depth in the sediment. Here, the methane is isotopically light ( δ13C < −60‰), which indicates that it is derived from the microbial reduction of CO2. The clathrates in these deep deposits are thought to have formed in situ from the microbially produced methane since the δ13C values of clathrate and surrounding dissolved methane are similar. However, it is also thought that freshwater used in the pressurization of oil and gas wells in permafrost and along the continental shelves worldwide combines with natural methane to form clathrate at depth and pressure since methane hydrates are more stable in freshwater than in saltwater. Local variations may be widespread since the act of forming hydrate, which extracts pure water from saline formation waters, can often lead to local and potentially significant increases in formation water salinity. Hydrates normally exclude the salt in the pore fluid from which it forms. Thus, they exhibit high electric resistivity like ice, and sediments containing hydrates have higher resistivity than sediments without gas hydrates (Judge 7.
These deposits are located within a mid-depth zone around 300–500 m thick in the sediments (the gas hydrate stability zone, or GHSZ) where they coexist with methane dissolved in the fresh, not salt, pore-waters. Above this zone methane is only present in its dissolved form at concentrations that decrease towards the sediment surface. Below it, methane is gaseous. At Blake Ridge on the Atlantic continental rise, the GHSZ started at 190 m depth and continued to 450 m, where it reached equilibrium with the gaseous phase. Measurements indicated that methane occupied 0-9% by volume in the GHSZ, and ~12% in the gaseous zone.
In the less common second type found near the sediment surface, some samples have a higher proportion of longer-chain hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s (< 99% methane) contained in a structure II clathrate. Carbon from this type of clathrate is isotopically heavier ( δ13C is −29 to −57 ‰) and is thought to have migrated upwards from deep sediments, where methane was formed by thermal decomposition of organic matter
Organic matter, organic material or natural organic matter is the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come fro ...
. Examples of this type of deposit have been found in the Gulf of Mexico
The Gulf of Mexico () is an oceanic basin and a marginal sea of the Atlantic Ocean, mostly surrounded by the North American continent. It is bounded on the northeast, north, and northwest by the Gulf Coast of the United States; on the southw ...
and the Caspian Sea
The Caspian Sea is the world's largest inland body of water, described as the List of lakes by area, world's largest lake and usually referred to as a full-fledged sea. An endorheic basin, it lies between Europe and Asia: east of the Caucasus, ...
.
Some deposits have characteristics intermediate between the microbially and thermally sourced types and are considered formed from a mixture of the two.
The methane in gas hydrates is dominantly generated by microbial consortia degrading organic matter in low oxygen environments, with the methane itself produced by methanogen
Methanogens are anaerobic archaea that produce methane as a byproduct of their energy metabolism, i.e., catabolism. Methane production, or methanogenesis, is the only biochemical pathway for Adenosine triphosphate, ATP generation in methanogens. A ...
ic archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
. Organic matter in the uppermost few centimeters of sediments is first attacked by aerobic bacteria, generating CO2, which escapes from the sediments into the water column
The (oceanic) water column is a concept used in oceanography to describe the physical (temperature, salinity, light penetration) and chemical ( pH, dissolved oxygen, nutrient salts) characteristics of seawater at different depths for a defined ...
. Below this region of aerobic activity, anaerobic processes take over, including, successively with depth, the microbial reduction of nitrite/nitrate, metal oxides, and then sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
s are reduced to sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s. Finally, methanogenesis becomes a dominant pathway for organic carbon remineralization.
If the sedimentation rate is low (about 1 cm/yr), the organic carbon content is low (about 1% ), and oxygen is abundant, aerobic bacteria can use up all the organic matter in the sediments faster than oxygen is depleted, so lower-energy electron acceptors are not used. But where sedimentation rates and the organic carbon content are high, which is typically the case on continental shelves and beneath western boundary current upwelling zones, the pore water in the sediments becomes anoxic
Anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of dissolved ox ...
at depths of only a few centimeters or less. In such organic-rich marine sediments, sulfate becomes the most important terminal electron acceptor due to its high concentration in seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
. However, it too is depleted by a depth of centimeters to meters. Below this, methane is produced. This production of methane is a rather complicated process, requiring a highly reducing environment (Eh −350 to −450 mV) and a pH between 6 and 8, as well as a complex syntrophic, consortia of different varieties of archaea and bacteria. However, it is only archaea that actually emit methane.
In some regions (e.g., Gulf of Mexico, Joetsu Basin) methane in clathrates may be at least partially derive from thermal degradation of organic matter (e.g. petroleum generation), with oil even forming an exotic component within the hydrate itself that can be recovered when the hydrate is disassociated. The methane in clathrates typically has a biogenic isotopic signature and highly variable δ13C (−40 to −100‰), with an approximate average of about −65‰ . Below the zone of solid clathrates, large volumes of methane may form bubbles of free gas in the sediments.
The presence of clathrates at a given site can often be determined by observation of a "bottom simulating reflector" (BSR), which is a seismic reflection at the sediment to clathrate stability zone interface caused by the unequal densities of normal sediments and those laced with clathrates.
Gas hydrate pingos have been discovered in the Arctic oceans Barents sea. Methane is bubbling from these dome-like structures, with some of these gas flares extending close to the sea surface.
Reservoir size
 The size of the oceanic methane clathrate reservoir is poorly known, and estimates of its size decreased by roughly an
The size of the oceanic methane clathrate reservoir is poorly known, and estimates of its size decreased by roughly an order of magnitude
In a ratio scale based on powers of ten, the order of magnitude is a measure of the nearness of two figures. Two numbers are "within an order of magnitude" of each other if their ratio is between 1/10 and 10. In other words, the two numbers are ...
per decade since it was first recognized that clathrates could exist in the oceans during the 1960s and 1970s. The highest estimates (e.g. 3 m3) were based on the assumption that fully dense clathrates could litter the entire floor of the deep ocean. Improvements in our understanding of clathrate chemistry and sedimentology have revealed that hydrates form in only a narrow range of depths (continental shelves
A continental shelf is a portion of a continent that is submerged under an area of relatively shallow water, known as a shelf sea. Much of these shelves were exposed by drops in sea level during glacial periods. The shelf surrounding an island ...
), at only some locations in the range of depths where they could occur (10-30% of the Gas hydrate stability zone), and typically are found at low concentrations (0.9–1.5% by volume) at sites where they do occur. Recent estimates constrained by direct sampling suggest the global inventory occupies between . This estimate, corresponding to 500–2500 gigatonnes carbon (Gt C), is smaller than the 5000 Gt C estimated for all other geo-organic fuel reserves but substantially larger than the ~230 Gt C estimated for other natural gas sources.USGS World Energy Assessment Team, 2000. US Geological Survey world petroleum assessment 2000––description and results. USGS Digital Data Series DDS-60. The permafrost reservoir has been estimated at about 400 Gt C in the Arctic, but no estimates have been made of possible Antarctic reservoirs. These are large amounts. In comparison, the total carbon in the atmosphere is around 800 gigatons (see ).
These modern estimates are notably smaller than the 10,000 to 11,000 Gt C (2 m3) proposed by previous researchers as a reason to consider clathrates to be a geo-organic fuel resource (MacDonald 1990, Kvenvolden 1998). Lower abundances of clathrates do not rule out their economic potential, but a lower total volume and apparently low concentration at most sites does suggest that only a limited percentage of clathrates deposits may provide an economically viable resource.
Continental
Methane clathrates in continental rocks are trapped in beds ofsandstone
Sandstone is a Clastic rock#Sedimentary clastic rocks, clastic sedimentary rock composed mainly of grain size, sand-sized (0.0625 to 2 mm) silicate mineral, silicate grains, Cementation (geology), cemented together by another mineral. Sand ...
or siltstone
Siltstone, also known as aleurolite, is a clastic sedimentary rock that is composed mostly of silt. It is a form of mudrock with a low clay mineral content, which can be distinguished from shale by its lack of fissility.
Although its permeabil ...
at depths of less than 800 m. Sampling indicates they are formed from a mix of thermally and microbially derived gas from which the heavier hydrocarbons were later selectively removed. These occur in Alaska
Alaska ( ) is a non-contiguous U.S. state on the northwest extremity of North America. Part of the Western United States region, it is one of the two non-contiguous U.S. states, alongside Hawaii. Alaska is also considered to be the north ...
, Siberia
Siberia ( ; , ) is an extensive geographical region comprising all of North Asia, from the Ural Mountains in the west to the Pacific Ocean in the east. It has formed a part of the sovereign territory of Russia and its predecessor states ...
, and Northern Canada
Northern Canada (), colloquially the North or the Territories, is the vast northernmost region of Canada, variously defined by geography and politics. Politically, the term refers to the three Provinces_and_territories_of_Canada#Territories, terr ...
.
In 2008, Canadian and Japanese researchers extracted a constant stream of natural gas from a test project at the Mallik gas hydrate site in the Mackenzie River
The Mackenzie River (French: ; Slavey language, Slavey: ' èh tʃʰò literally ''big river''; Inuvialuktun: ' uːkpɑk literally ''great river'') is a river in the Canadian Canadian boreal forest, boreal forest and tundra. It forms, ...
delta. This was the second such drilling at Mallik: the first took place in 2002 and used heat to release methane. In the 2008 experiment, researchers were able to extract gas by lowering the pressure, without heating, requiring significantly less energy. The Mallik gas hydrate field was first discovered by Imperial Oil in 1971–1972.
Commercial use
Economic deposits of hydrate are termed natural gas hydrate (NGH) and store 164 m3 of methane, 0.8 m3 water in 1 m3 hydrate. Most NGH is found beneath the seafloor (95%) where it exists in thermodynamic equilibrium. The sedimentary methane hydrate reservoir probably contains 2–10 times the currently known reserves of conventionalnatural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
, .
This represents a potentially important future source of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied solely to those materials capable of releasing chem ...
. However, in the majority of sites deposits are thought to be too dispersed for economic extraction. Other problems facing commercial exploitation are detection of viable reserves and development of the technology for extracting methane gas from the hydrate deposits.
In August 2006, China announced plans to spend 800 million yuan (US$100 million) over the next 10 years to study natural gas hydrates. A potentially economic reserve in the Gulf of Mexico may contain approximately of gas. Bjørn Kvamme and Arne Graue at the Institute for Physics and technology at the University of Bergen
The University of Bergen () is a public university, public research university in Bergen, Norway. As of 2021, the university had over 4,000 employees and 19,000 students. It was established by an act of parliament in 1946 consolidating several sci ...
have developed a method for injecting into hydrates and reversing the process; thereby extracting CH4 by direct exchange. The University of Bergen's method is being field tested by ConocoPhillips and state-owned Japan Oil, Gas and Metals National Corporation (JOGMEC), and partially funded by the U.S. Department of Energy. The project has already reached injection phase and was analyzing resulting data by March 12, 2012.
On March 12, 2013, JOGMEC researchers announced that they had successfully extracted natural gas from frozen methane hydrate. In order to extract the gas, specialized equipment was used to drill into and depressurize the hydrate deposits, causing the methane to separate from the ice. The gas was then collected and piped to surface where it was ignited to prove its presence. According to an industry spokesperson, "It asthe world's first offshore experiment producing gas from methane hydrate". Previously, gas had been extracted from onshore deposits, but never from offshore deposits which are much more common. The hydrate field from which the gas was extracted is located from central Japan in the Nankai Trough
The is a submarine trough located south of the Nankaidō region of Japan's island of Honshu, extending approximately offshore. The underlying fault, the ''Nankai megathrust,'' is the source of the devastating Nankai megathrust earthquakes, ...
, under the sea. A spokesperson for JOGMEC remarked "Japan could finally have an energy source to call its own". Marine geologist Mikio Satoh remarked "Now we know that extraction is possible. The next step is to see how far Japan can get costs down to make the technology economically viable." Japan estimates that there are at least 1.1 trillion cubic meters of methane trapped in the Nankai Trough, enough to meet the country's needs for more than ten years.
Both Japan and China announced in May 2017 a breakthrough for mining
Mining is the Resource extraction, extraction of valuable geological materials and minerals from the surface of the Earth. Mining is required to obtain most materials that cannot be grown through agriculture, agricultural processes, or feasib ...
methane clathrates, when they extracted methane from hydrates in the South China Sea
The South China Sea is a marginal sea of the Western Pacific Ocean. It is bounded in the north by South China, in the west by the Indochinese Peninsula, in the east by the islands of Taiwan island, Taiwan and northwestern Philippines (mainly Luz ...
. China described the result as a breakthrough; Praveen Linga from the Department of Chemical and Biomolecular Engineering at the National University of Singapore agreed "Compared with the results we have seen from Japanese research, the Chinese scientists have managed to extract much more gas in their efforts". Industry consensus is that commercial-scale production remains years away.
Environmental concerns
Experts caution that environmental impacts are still being investigated and that methane—a greenhouse gas with around 86 times as muchglobal warming potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide ( ...
over a 20-year period (GWP100) as carbon dioxide—could potentially escape into the atmosphere if something goes wrong. Furthermore, while cleaner than coal, burning natural gas also creates carbon dioxide emissions.
Hydrates in natural gas processing
Routine operations
Methane clathrates (hydrates) are also commonly formed during natural gas production operations, when liquid water is condensed in the presence of methane at high pressure. It is known that larger hydrocarbon molecules like ethane and propane can also form hydrates, although longer molecules (butanes, pentanes) cannot fit into the water cage structure and tend to destabilise the formation of hydrates. Once formed, hydrates can block pipeline and processing equipment. They are generally then removed by reducing the pressure, heating them, or dissolving them by chemical means (methanol is commonly used). Care must be taken to ensure that the removal of the hydrates is carefully controlled, because of the potential for the hydrate to undergo aphase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
from the solid hydrate to release water and gaseous methane at a high rate when the pressure is reduced. The rapid release of methane gas in a closed system can result in a rapid increase in pressure.
It is generally preferable to prevent hydrates from forming or blocking equipment. This is commonly achieved by removing water, or by the addition of ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
(MEG) or methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
, which act to depress the temperature at which hydrates will form. In recent years, development of other forms of hydrate inhibitors have been developed, like Kinetic Hydrate Inhibitors (increasing the required sub-cooling which hydrates require to form, at the expense of increased hydrate formation rate) and anti-agglomerates, which do not prevent hydrates forming, but do prevent them sticking together to block equipment.
Effect of hydrate phase transition during deep water drilling
When drilling in oil- and gas-bearing formations submerged in deep water, the reservoir gas may flow into the well bore and form gas hydrates owing to the low temperatures and high pressures found during deep water drilling. The gas hydrates may then flow upward with drilling mud or other discharged fluids. When the hydrates rise, the pressure in the annulus decreases and the hydrates dissociate into gas and water. The rapid gas expansion ejects fluid from the well, reducing the pressure further, which leads to more hydrate dissociation and further fluid ejection. The resulting violent expulsion of fluid from the annulus is one potential cause or contributor to the "kick". (Kicks, which can cause blowouts, typically do not involve hydrates: see ). Measures which reduce the risk of hydrate formation include: * High flow-rates, which limit the time for hydrate formation in a volume of fluid, thereby reducing the kick potential. * Careful measuring of line flow to detect incipient hydrate plugging. * Additional care in measuring when gas production rates are low and the possibility of hydrate formation is higher than at relatively high gas flow rates. * Monitoring of well casing after it is " shut in" (isolated) may indicate hydrate formation. Following "shut in", the pressure rises while gas diffuses through the reservoir to the bore hole; the rate of pressure rise exhibit a reduced rate of increase while hydrates are forming. * Additions of energy (e.g., the energy released by setting cement used in well completion) can raise the temperature and convert hydrates to gas, producing a "kick".Blowout recovery
 At sufficient depths, methane complexes directly with water to form methane hydrates, as was observed during the
At sufficient depths, methane complexes directly with water to form methane hydrates, as was observed during the Deepwater Horizon oil spill
The ''Deepwater Horizon'' oil spill was an environmental disaster off the coast of the United States in the Gulf of Mexico, on the BP-operated Macondo Prospect. It is considered the largest marine oil spill in the history of the petroleum in ...
in 2010. BP engineers developed and deployed a subsea oil recovery system over oil spilling from a deepwater oil well
An oil well is a drillhole boring in Earth that is designed to bring petroleum oil hydrocarbons to the surface. Usually some natural gas is released as associated petroleum gas along with the oil. A well that is designed to produce only gas m ...
below sea level
Mean sea level (MSL, often shortened to sea level) is an mean, average surface level of one or more among Earth's coastal Body of water, bodies of water from which heights such as elevation may be measured. The global MSL is a type of vertical ...
to capture escaping oil. This involved placing a dome over the largest of the well leaks and piping it to a storage vessel on the surface. This option had the potential to collect some 85% of the leaking oil but was previously untested at such depths. BP deployed the system on May 7–8, but it failed due to a buildup of methane clathrate inside the dome; with its low density of approximately 0.9 g/cm3 the methane hydrates accumulated in the dome, adding buoyancy and obstructing flow.
Methane clathrates and climate change
Natural gas hydrates for gas storage and transportation
Since methane clathrates are stable at a higher temperature than liquefied natural gas (LNG) (−20 vs −162 °C), there is some interest in converting natural gas into clathrates (Solidified Natural Gas or SNG) rather than liquifying it when transporting it by seagoing vessels. A significant advantage would be that the production of natural gas hydrate (NGH) from natural gas at the terminal would require a smaller refrigeration plant and less energy than LNG would. Offsetting this, for 100 tonnes of methane transported, 750 tonnes of methane hydrate would have to be transported; since this would require a ship of 7.5 times greater displacement, or require more ships, it is unlikely to prove economically feasible.. Recently, methane hydrate has received considerable interest for large scale stationary storage application due to the very mild storage conditions with the inclusion oftetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) as a co-guest. With the inclusion of tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, though there is a slight reduction in the gas storage capacity, the hydrates have been demonstrated to be stable for several months in a recent study at −2 °C and atmospheric pressure. A recent study has demonstrated that SNG can be formed directly with seawater instead of pure water in combination with THF.
See also
*Future energy development
The future is the time after the past and present. Its arrival is considered inevitable due to the existence of time and the laws of physics. Due to the apparent nature of reality and the unavoidability of the future, everything that currently ...
* Long-term effects of global warming
* ''The Swarm'' (Schätzing novel)
*Unconventional (oil & gas) reservoir
Unconventional (oil and gas) reservoirs, or unconventional resources (resource plays) are accumulations where oil and gas phases are tightly bound to the rock fabric by strong capillary forces, requiring specialized measures for evaluation and ...
Notes
References
External links
Are there deposits of methane under the sea? Will global warming release the methane to the atmosphere?
(2007)
Methane seeps from Arctic sea bed
(BBC)
Bubbles of warming, beneath the ice
(LA Times 2009)
online calculator : hydrate formation conditions with different EOSs
Research
Centre for Arctic Gas Hydrate, Environment and Climate (CAGE)
* ttps://web.archive.org/web/20070105164147/http://www.eee.columbia.edu/research-projects/sustainable_energy/Hydrates/index.html Carbon Neutral Methane Energy Production from Hydrate Deposits(Columbia University)
Video
USGS Gas Hydrates Lab
(2012)
Ancient Methane Explosions Created Ocean Craters
(2017) {{DEFAULTSORT:Methane Clathrate Clathrate hydrates Hydrocarbons Methane Unconventional gas Natural gas