Kedarcidin on:
[Wikipedia]
[Google]
[Amazon]
Kedarcidin is a


 The unifying mechanism of bioactivity in all enediyne antibiotics is the
The unifying mechanism of bioactivity in all enediyne antibiotics is the 
 Recent evidence suggests that spontaneous cycloaromatization of kedarcidin chromophore is competitive with nucleophilic bioactivation, if not the predominant mechanism ''in vivo''. While MM2 calculations show that the C1–C12 double bond in the bicyclic core imparts a considerable amount of ring strain (ca. 14 kcal·mol−1) to the ,5,5tricycle formed upon Bergman cyclization–reduction, Hirama ''et al.'' note that the 5,9-fused enediyne core is susceptible to cycloaromatization–reduction in the absence of both thiol "activating agents" and (non-solvent) hydrogen donors. The kedarcidin chromophore aglycone similarly undergoes reductive cycloaromatization at comparable rates irrespective of the presence of
Recent evidence suggests that spontaneous cycloaromatization of kedarcidin chromophore is competitive with nucleophilic bioactivation, if not the predominant mechanism ''in vivo''. While MM2 calculations show that the C1–C12 double bond in the bicyclic core imparts a considerable amount of ring strain (ca. 14 kcal·mol−1) to the ,5,5tricycle formed upon Bergman cyclization–reduction, Hirama ''et al.'' note that the 5,9-fused enediyne core is susceptible to cycloaromatization–reduction in the absence of both thiol "activating agents" and (non-solvent) hydrogen donors. The kedarcidin chromophore aglycone similarly undergoes reductive cycloaromatization at comparable rates irrespective of the presence of

 The bicyclic core of C10-epi-kedarcidin chromophore was prepared by the sequential application of three carbon-carbon bond forming reactions, as shown in the retrosynthetic schematic above. First, a
The bicyclic core of C10-epi-kedarcidin chromophore was prepared by the sequential application of three carbon-carbon bond forming reactions, as shown in the retrosynthetic schematic above. First, a
 The biosynthetic gene clusters encoding the biological machinery responsible for producing enediynes have been cloned and characterized for five 9-membered enediynes (
The biosynthetic gene clusters encoding the biological machinery responsible for producing enediynes have been cloned and characterized for five 9-membered enediynes ( The 2-aza-β-tyrosine subunit of kedarcidin chromophore is altogether unknown in any other natural product; this lack of precedence frustrates any attempt at ''a priori'' identification of the genes responsible for synthesizing this structure. However, six genes are conserved among the biosynthetic clusters of kedarcidin,
The 2-aza-β-tyrosine subunit of kedarcidin chromophore is altogether unknown in any other natural product; this lack of precedence frustrates any attempt at ''a priori'' identification of the genes responsible for synthesizing this structure. However, six genes are conserved among the biosynthetic clusters of kedarcidin,  Insight into the biosynthesis of the isopropoxy-2-naphthonate appendage was similarly gained by comparative analysis of the ''ked'' cluster to those of
Insight into the biosynthesis of the isopropoxy-2-naphthonate appendage was similarly gained by comparative analysis of the ''ked'' cluster to those of
Kedarcidin, a new chromoprotein antitumor antibiotic. II. Isolation, purification and physico-chemical properties
{{Enediynes Antineoplastic drugs Epoxides Cancer research Enediynes Naphthalenes Experimental drugs Lactones Isopropyl compounds Nine-membered rings
chromoprotein A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes Hemoglobin#Oxyhemoglobin, oxygenated ...
antitumor antibiotic
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) in a standard regimen. Chemotherapy may be given with a curat ...
first isolated from an Actinomycete
The Actinomycetales is an order of Actinomycetota. A member of the order is often called an actinomycete. Actinomycetales are generally gram-positive and anaerobic and have mycelia in a filamentous and branching growth pattern. Some actinomycetes ...
in 1992, comprising an ansa-bridged enediyne
Enediynes are organic compounds containing two triple bonds and one double bond.
Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in c ...
chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne
Enediynes are organic compounds containing two triple bonds and one double bond.
Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in c ...
class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
directly attached to two alkynyl
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer An anticarcinogen (also known as a carcinopreventive agent) is a substance that counteracts the effects of a carcinogen or inhibits the development of cancer. Anticarcinogens are different from anticarcinoma agents (also known as anticancer or ant ...
properties.
Discovery and structure elucidation
Kedarcidin was first discovered in 1992 when bioassays conducted atBristol-Myers Squibb
The Bristol-Myers Squibb Company, doing business as Bristol Myers Squibb (BMS), is an American multinational pharmaceutical company. Headquartered in Princeton, New Jersey, BMS is one of the world's largest pharmaceutical companies and consist ...
indicated the presence of a DNA-damaging chromoprotein in the fermentation broth of an Actinomycete strain. The involvement of a non-peptidic chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
was deduced by UV spectroscopy, and reverse-phase chromatography was used to separate this noncovalently bound chromophore from its apoprotein host. This isolate—kedarcidin chromophore—decomposed readily under ambient conditions and was shown to possess cytotoxicity ( IC50 0.4 ng/ml, HCT-116 human colorectal carcinoma cell line).
Subsequent NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
, mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
, chemical degradation, and derivatization experiments enabled the isolation team to identify the key structural features of kedarcidin chromophore, including the enediyne bicyclic core, the ansa-bridging chloropyridyl ring, the mycarose and kedarosamine sugars, and the naphthoamide appendage. However, due to the challenges posed by the complex structure, the initial report had several errors. The bicyclic core proved particularly difficult to deconvolute, as the interpretation of NOE correlations led the researchers to misassign the relative stereochemistry of the core stereotetrad. Moreover, as global absolute chemistry was assigned on the basis of NOE correlations between the stereodefined L-mycarose sugar and the aglycone
An aglycone (aglycon or genin) is the chemical compound remaining after the glycosyl group on a glycoside is replaced by a hydrogen atom. For example, the aglycone of a cardiac glycoside would be a steroid
A steroid is an organic compoun ...
, the errors of the stereotetrad propagated to the other two stereocenters of the aglycone. Connectivity of the naphthoamide group to the ''ansa'' bridge was also misjudged in the initial report.
These errors were later corrected by the independent synthetic efforts of researchers at Tohoku University
is a public research university in Sendai, Miyagi, Japan. It is colloquially referred to as or .
Established in 1907 as the third of the Imperial Universities, after the University of Tokyo and Kyoto University, it initially focused on sc ...
and Harvard University
Harvard University is a Private university, private Ivy League research university in Cambridge, Massachusetts, United States. Founded in 1636 and named for its first benefactor, the History of the Puritans in North America, Puritan clergyma ...
. In 1997, en route to the originally reported structure, researchers under the direction of Masahiro Hirama discovered that the spectroscopic data of the proposed chloroazatyrosyl (''S'')-α-amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
derivative were not consistent with those of the degradation product characterized by Leet ''et al''. Instead, an (''R'')-β-amino acid
Beta-peptides (β-peptides) are peptides derived from β-amino acids, in which the amino group is attached to the β-carbon (i.e. the carbon two atoms away from the carboxylate group). The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a co ...
derivative was proposed and validated by the Hirama group. This revision led Hirama ''et al''. to invert the other aglycone stereocenters as well, affording a revised structure of kedarcidin chromophore that differed only in the relative stereochemistry of the mycarose-bearing carbon, C10. Finally, in 2007, Myers and co-workers synthesized the structure proposed by Hirama ''et al''.; the corresponding NMR spectroscopic data were distinct from that of the natural product, leading the Myers group to revise the stereochemistry of the mycarose-bearing carbon to 10-(''S'').

Mechanism of action
Like other enediynes, kedarcidin chromophore comprises a core structure that forms destructive free radicals, as well as appendages that deliver this "warhead" to its DNA target. Thus, the general mechanism by which kedarcidin chromophore damages DNA is known; however, the details of this process—particularly the necessity of nucleophilic activation—have been disputed.
Free-radical DNA damage
 The unifying mechanism of bioactivity in all enediyne antibiotics is the
The unifying mechanism of bioactivity in all enediyne antibiotics is the Bergman cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen ...
, wherein the enediyne portion undergoes spontaneous cycloaromatization to generate a ''para''- benzyne biradical activated toward homolytic abstraction of hydrogen from suitable donors, including the deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
sugars of DNA. This generates a carbon-centered free radical on DNA, which undergoes oxidation by molecular oxygen. The resulting peroxide decomposes to form single- or double-stranded breaks in DNA, ultimately leading to cell death.
With considerable sequence selectivity, kedarcidin chromophore binds and cleaves DNA preferentially at TCCTn-mer sites, producing single-strand breaks. Puzzlingly, while the structure of kedarcidin chromophore is most closely related to that of neocarzinostatin chromophore, the former shares sequence-specificity with the structurally distinct calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium '' Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "cal ...
enediyne antitumor antibiotic. The naphthoic acid substructure has been implicated in DNA binding, likely through intercalation. To this end, kedarcidin chromophore–induced DNA cleavage is diminished by the addition of divalent cations such as Ca2+ and Mg2+, which chelatively bind the naphthoic acid group of kedarcidin chromophore and thus lessen its affinity for DNA. Competition experiments with netropsin
Netropsin (also termed congocidine or sinanomycin) is a polyamide with antibiotic and antiviral activity. Netropsin was discovered by Finlay ''et al.'', and first isolated from the actinobacterium '' Streptomyces netropsis''. It belongs to the cl ...
, a known binder of the DNA minor groove, indicate that kedarcidin likely binds the minor groove as well.

Nucleophilic activation
''In vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
'' nucelophilic addition of thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s to C12 and consequent opening of the core epoxide has been hypothesized to trigger Bergman cyclization in kedarcidin chromophore. Nucleophilic activation is thought to diminish the ring strain incurred by formation of the cycloaromatized product, and thus activate kedarcidin chromophore toward DNA scission. In the isolation and structural characterization studies carried out by Leet ''et al''., C12-sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
reduction of kedarcidin chromophore induced rapid cycloaromatization and so facilitated studies of the otherwise unstable natural product. Consequently, C12-nucleophilic activation is proposed extensively in review literature as a possible means for triggering the cycloaromatization event ''in vivo''.
 Recent evidence suggests that spontaneous cycloaromatization of kedarcidin chromophore is competitive with nucleophilic bioactivation, if not the predominant mechanism ''in vivo''. While MM2 calculations show that the C1–C12 double bond in the bicyclic core imparts a considerable amount of ring strain (ca. 14 kcal·mol−1) to the ,5,5tricycle formed upon Bergman cyclization–reduction, Hirama ''et al.'' note that the 5,9-fused enediyne core is susceptible to cycloaromatization–reduction in the absence of both thiol "activating agents" and (non-solvent) hydrogen donors. The kedarcidin chromophore aglycone similarly undergoes reductive cycloaromatization at comparable rates irrespective of the presence of
Recent evidence suggests that spontaneous cycloaromatization of kedarcidin chromophore is competitive with nucleophilic bioactivation, if not the predominant mechanism ''in vivo''. While MM2 calculations show that the C1–C12 double bond in the bicyclic core imparts a considerable amount of ring strain (ca. 14 kcal·mol−1) to the ,5,5tricycle formed upon Bergman cyclization–reduction, Hirama ''et al.'' note that the 5,9-fused enediyne core is susceptible to cycloaromatization–reduction in the absence of both thiol "activating agents" and (non-solvent) hydrogen donors. The kedarcidin chromophore aglycone similarly undergoes reductive cycloaromatization at comparable rates irrespective of the presence of β-mercaptoethanol
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by sc ...
, a common thiol reductant. In a model system, it was found that the 5,9-bicyclic core of kedarcidin chromophore exists in equilibrium with the corresponding 5,5,6-tricyclic cycloaromatized biradical. The rate of pseudo-first-order decay of this model enediyne is highly dependent on the solvent hydrogen-donor ability, indicating that the hydrogen abstraction step following biradical formation is kinetically significant in the cycloaromatization of the enediyne, as opposed to acyclic systems, where formation of the biradical itself is known to be the rate-limiting step. It is noteworthy that of the solvents examined, tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
—structurally homologous with deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
—led to comparatively fast decomposition of the 5,9-fused enediyne scaffold (''t''½ = 68 min); Zein ''et al.'' independently remark that deoxyribose 4'-hydrogen abstraction is most likely operative in kedarcidin chromophore bioactivity.
Synthesis of epi-kedarcidin chromophore
In 2007, Myers and co-workers at Harvard University reported the synthesis of C10-epi-kedarcidin chromophore, corresponding to the 1997 revised structure advanced by Hirama ''et al''. Critical to the success of this endeavor wasretrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. ...
that focused on the convergent coupling of components with roughly equal chemical complexity. Several of the major challenges of C10-epi-kedarcidin chromophore, as well as the strategies used in addressing these difficulties are discussed below.

Inherent instability of the enediyne core
The instability towardBergman cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen ...
–reduction decomposition pathways poses a major threat to any proposed synthesis of enediyne
Enediynes are organic compounds containing two triple bonds and one double bond.
Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in c ...
s. Myers and co-workers addressed this liability by late-stage dehydrative installation of the olefin. Without this unsaturation linking the two alkynyl bridges, synthetic intermediates are not disposed toward Bergman-type decomposition, and risk of decomposition is mitigated. In this case, dehydration of a propargyl
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework ...
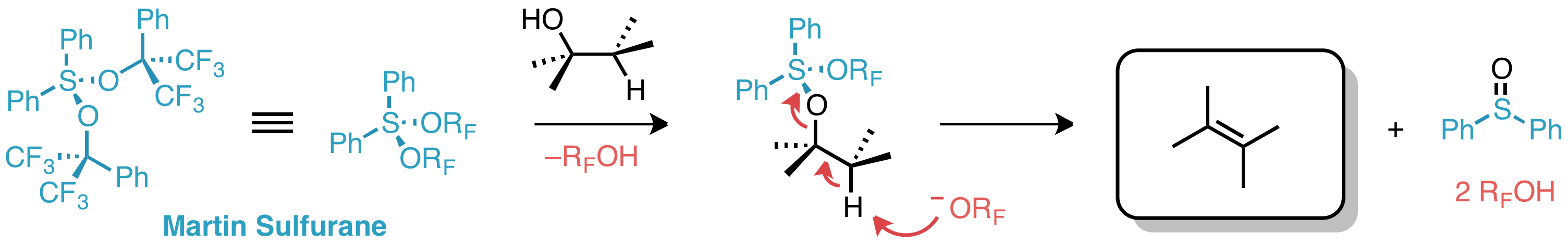
ic alcohol was induced by treatment with Martin sulfurane.

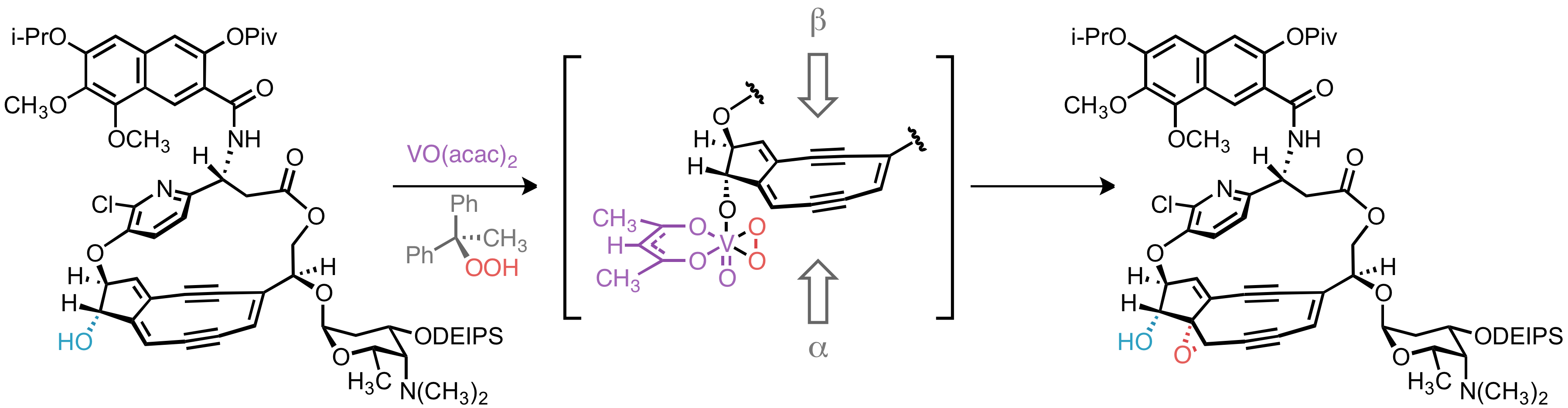
Epoxide stereochemistry
In targeting 10-epi-kedarcidin chromophore, Myers ''et al''. sought to install the epoxide functionality syn to the adjacent C10 hydroxyl group. This was accomplished by vanadium-catalyzed epoxidation directed by the C10 hydroxyl group.Rossiter, B. E.; Verhoeven, T. R.; Sharpless, K. B. ''Tetrahedron Lett.'' 1979, ''20'', 4733. Had the natural C10-(''S'')-epimer been desired, it is conceivable that trialkylsilyl protection of the C10 hydroxyl would lead to the desired α-face epoxidation product by steric occlusion of the β face of the olefin; however, without a directing group to accelerate the oxidation of a proximal alkene, this hypothetical reaction would likely suffer from poorregioselectivity
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
, as oxidation of other C–C unsaturations in the molecule would compete with the desired reaction.

Construction of the bicyclic core
Myers and co-workers have pioneered the application of transannular anionic cyclization reactions in the synthesis of the 5,9-fused bicyclic core of kedarcidin chromophore and neocarzinostatin chromophore. In the first incarnation, hydride delivery to a cyclic tetrayne was guided by aluminum coordination to a proximal alkoxide, thus generating the desired enediyne core in one step via two successive 5-exo-dig–type cyclizations. Later-generation syntheses of the core intercept this cascade cyclization, relying on lithium-halogen exchange on a cyclic vinyl bromide to generate the vinyl anion precursor to the bicyclic product. The bicyclic core of C10-epi-kedarcidin chromophore was prepared by the sequential application of three carbon-carbon bond forming reactions, as shown in the retrosynthetic schematic above. First, a
The bicyclic core of C10-epi-kedarcidin chromophore was prepared by the sequential application of three carbon-carbon bond forming reactions, as shown in the retrosynthetic schematic above. First, a Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
was carried out between a bromovinyl electrophile and alkynyl nucleophile; ring closure to give a cyclic triyne was then accomplished by Glaser coupling
The Glaser coupling is a type of coupling reaction. It is by far one of the oldest coupling reactions and is based on copper compounds like copper(I) chloride or copper(I) bromide and an additional oxidant like air. The base used in the original ...
of two terminal alkynes. The 5,9-fused bicyclic core was established by ''in situ'' generation of a vinyllithium
Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in organic synthesis, synthesis of organic compounds, especially for ...
species that underwent transannular 5-exo-dig cyclization.
Ansa-bridging macrolactone
The ansa-bridging macrolactone was constructed following the first Sonogashira coupling, using the Shiina macrolactonization. This protocol was performed on the gram-scale without diminishing its yield employing 2-methyl-6-nitrobenzoic anhydride, 4-dimethylaminopyridine, andtriethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
as a base to promote intramolecular esterification.
Biosynthesis
The means by which bacteria construct enediynes like kedarcidin continues to motivate research. Kedarcidin chromophore, beyond the carbocyclic core it shares with other enediynes, presents additionalbiosynthetic
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme- catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve ...
puzzles: The relative stereochemistry of the groups appended to the carbocyclic core of kedarcidin chomophore differs from that of closely related enediynes; the (''R'')-2-aza-3-chloro-β-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
substructure has not been identified in any other known natural product; and despite its seeming simplicity, little literature precedence exists for the biosynthesis of the isopropoxy substituent of the naphthonate group.
 The biosynthetic gene clusters encoding the biological machinery responsible for producing enediynes have been cloned and characterized for five 9-membered enediynes (
The biosynthetic gene clusters encoding the biological machinery responsible for producing enediynes have been cloned and characterized for five 9-membered enediynes (C-1027
C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoenzyme, apoprotein.
It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules k ...
, neocarzinostatin
Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by ''Streptomyces macromomyceticus''.
It consists of two parts, a labile chromophore (the non-protein molecular entity shown at right) and a 113 am ...
, maduropeptin, sporolides
Sporolides A and B are polycyclic macrolides extracted from the obligate marine bacterium '' Salinispora tropica'', which is found in ocean sediment. They are composed of a chlorinated cyclopenta ndene ring and a cyclohexenone moiety. They were th ...
, and kedarcidin), and three 10-membered enediynes (calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium '' Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "cal ...
, esperamicin, and dynemicin). Comparative studies of these biosynthetic apparatus have shown that the enediyne core of these molecules is initiated by a common enzyme, enediyne polyketide synthase (PKS). The polyene product of this enzyme is then divergently elaborated into the 9- or 10-membered cores of the enediynes depending on the specific PKS-associated enzymes present. A convergent biosynthetic strategy is then employed by the producing organisms, whereby the varying peripheral appendages of the enediynes are attached to the core structure to furnish the final product.
In 2013, the successful cloning and characterization of the kedarcidin biosynthetic cluster ("''ked''") was reported by researchers at the Scripps Research Institute
Scripps Research is a nonprofit American medical research facility that focuses on research and education in the biomedical sciences. Headquartered in San Diego, California, the institute has over 170 laboratories employing 2,100 scientists, tec ...
and the University of Wisconsin-Madison
A university () is an institution of tertiary education and research which awards academic degrees in several academic disciplines. ''University'' is derived from the Latin phrase , which roughly means "community of teachers and scholars". Uni ...
. The identity of this cloned gene cluster was corroborated by ''kedA'', a gene in the cluster that encodes the previously isolated kedarcidin apoprotein, as well as ''kedE'' and ''kedE10'', the co-expression of which in ''E. coli'' led to the formation of a signature heptaene product previously implicated in enediyne core biosynthesis.
 The 2-aza-β-tyrosine subunit of kedarcidin chromophore is altogether unknown in any other natural product; this lack of precedence frustrates any attempt at ''a priori'' identification of the genes responsible for synthesizing this structure. However, six genes are conserved among the biosynthetic clusters of kedarcidin,
The 2-aza-β-tyrosine subunit of kedarcidin chromophore is altogether unknown in any other natural product; this lack of precedence frustrates any attempt at ''a priori'' identification of the genes responsible for synthesizing this structure. However, six genes are conserved among the biosynthetic clusters of kedarcidin, C-1027
C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoenzyme, apoprotein.
It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules k ...
, and maduropeptin—while these later two enediynes do not contain a 2-aza-β-tyrosine subunit, they do feature similar (L)-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
-derived components, leading Shen ''et al.'' to propose a pathway for the synthesis of the corresponding kedarcidin subunit beginning with 2-aza-L-tyrosine. This α-amino acid is thus believed to be converted to the corresponding β-amino acid by KedY4, an aminomutase encoded in the ''ked'' cluster. The resulting product is believed to be loaded onto the peptidyl carrier protein
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they ...
KedY2 and subsequently chlorinated by KedY3, an flavin adenine dinucleotide
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which ma ...
-dependent halogenase.
 Insight into the biosynthesis of the isopropoxy-2-naphthonate appendage was similarly gained by comparative analysis of the ''ked'' cluster to those of
Insight into the biosynthesis of the isopropoxy-2-naphthonate appendage was similarly gained by comparative analysis of the ''ked'' cluster to those of neocarzinostatin
Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by ''Streptomyces macromomyceticus''.
It consists of two parts, a labile chromophore (the non-protein molecular entity shown at right) and a 113 am ...
and maduropeptin, enediynes with naphthonate or benzoate
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
substructures, respectively. Five genes, KedN1–N5, bear high sequence homology with the enzymes responsible for naphthonate synthesis in neocarzinostatin—consequently, the intermediacy of 3,6,8-trihydroxy-2-naphthoic acid is proposed in kedarcidin biosynthesis. This compound is believed to be oxygenated to the 3,6,7,8-tetrahydroxy derivative, then triply ''O''-methylated by KedN1, an ''O''-methyltransferase. To furnish the unique isopropoxy substituent, Shen ''et al.'' invoke double ''C''-methylation of the corresponding methoxy group by the radical SAM
Radical SAM enzymes belong to a superfamily of enzymes that use an iron-sulfur cluster (4Fe-4S) to reductively cleave S-Adenosyl methionine, ''S''-adenosyl-L-methionine (SAM) to generate a radical (chemistry), radical, usually a 5′-deoxyadenosyl ...
methyltransferase
Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Ro ...
KedN5.
Conclusion
Owing to its non-specific cytotoxicity, instability under ambient conditions, and relative expense of isolation and manufacture, kedarcidin chromophore has not been investigated rigorously as a therapeutic candidate. However, the recent scientific advances discussed above have served to diminish this last hurdle, as fully synthetic andbiosynthetic
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme- catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve ...
routes toward scalable kedarcidin production are now within reach. Moreover, with the rising popularity of antibody-drug conjugate therapies, toxicity liabilities may be mitigated through targeted delivery of this potent cytotoxin, potentially enabling efficient therapies that use minimal quantities of this complex material. The recent development of inotuzumab ozogamicin
Inotuzumab ozogamicin, sold under the brand name Besponsa, is an antibody-drug conjugate medication used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). It is administered by intravenous infusion.
Inotuzum ...
, a calicheamicin-based antibody-drug conjugate for the treatment of non-Hodgkin lymphoma, reinforces the potential of enediynes to find critical use in the treatment of human disease. Thus, the biological potential and complex molecular architecture of kedarcidin may likely inspire further scientific inquiry into this substance, and possibly deliver new ordnance in the war against cancer.
References
External links
Kedarcidin, a new chromoprotein antitumor antibiotic. II. Isolation, purification and physico-chemical properties
{{Enediynes Antineoplastic drugs Epoxides Cancer research Enediynes Naphthalenes Experimental drugs Lactones Isopropyl compounds Nine-membered rings