Julia Olefination on:
[Wikipedia]
[Google]
[Amazon]
The Julia olefination (also known as the Julia–Lythgoe olefination) is the  The utility of this connective olefination reaction arises from its versatility, its wide functional group tolerance, and the mild reaction conditions under which the reaction proceeds.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is
The utility of this connective olefination reaction arises from its versatility, its wide functional group tolerance, and the mild reaction conditions under which the reaction proceeds.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is
 The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often. This transformation highly favors formation of the ''E''-alkene.
The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often. This transformation highly favors formation of the ''E''-alkene.
 The modified Julia olefination, also known as the one-pot Julia olefination is a modification of the classical Julia olefination. The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the
The modified Julia olefination, also known as the one-pot Julia olefination is a modification of the classical Julia olefination. The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the
 The Julia–Kocienski Olefination, a further refinement of the Modified Julia olefination, offers very good ''E''-selectivity. In the Julia–Kocienski olefination the
The Julia–Kocienski Olefination, a further refinement of the Modified Julia olefination, offers very good ''E''-selectivity. In the Julia–Kocienski olefination the 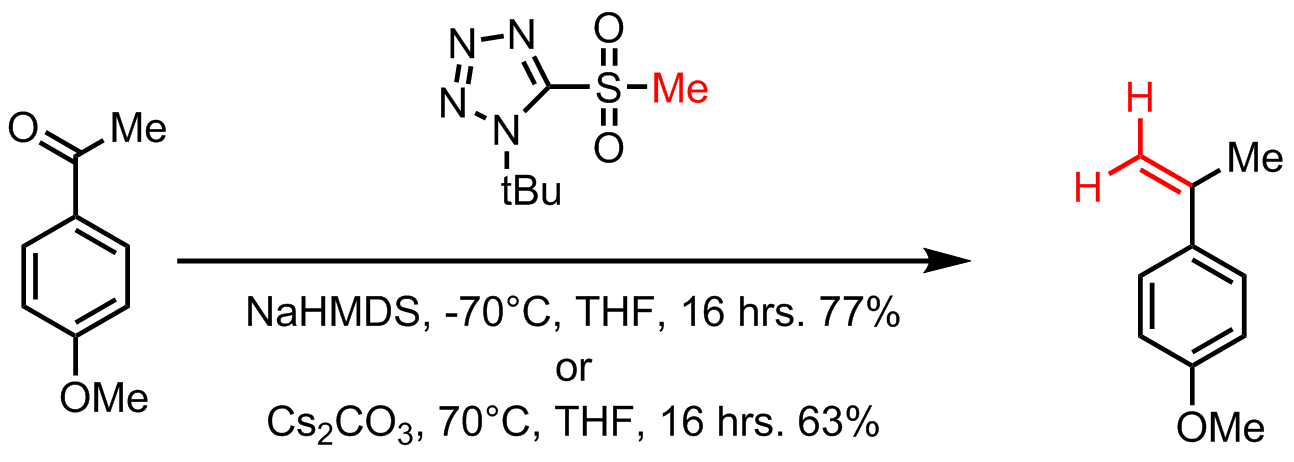



Julia-Lythgoe OlefinationJulia OlefinationJulia-Kocienski Olefination
{{Alkenes Olefination reactions Carbon-carbon bond forming reactions Addition reactions Free radical reactions Name reactions
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
used in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
of phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s (1) with aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
(or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s) to give alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam
Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. ...
or SmI2. The reaction is named after the French chemist Marc Julia
Marc Julia (23 October 1922 – 29 June 2010) was a French chemist and the winner of the 1990 CNRS Gold Medal in chemistry. He discovered the Julia olefination reaction in 1973.
Biography
Julia was born in 1922 in Paris as son of the renowned m ...
.
 The utility of this connective olefination reaction arises from its versatility, its wide functional group tolerance, and the mild reaction conditions under which the reaction proceeds.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is
The utility of this connective olefination reaction arises from its versatility, its wide functional group tolerance, and the mild reaction conditions under which the reaction proceeds.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is acetyl
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
or benzoyl
In organic chemistry, benzoyl (, ) is the functional group with the formula and structure . It can be viewed as benzaldehyde missing one hydrogen. The benzoyl group has a mass of 105 amu.
The term "benzoyl" should not be confused with benzyl ...
, with acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
or benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It is ...
used in the preparation of 2.
History
In 1973, Marc Julia and Jean-Marc Paris reported a novel olefin synthesis in which β-acyloxysulfones were reductively eliminated to the corresponding di-, tri-, or tetrasubstituted alkenes. Basil Lythgoe and Philip J. Kocienski explored the scope and limitation of the reaction, and today this olefination is formally known as the Julia-Lythgoe olefination. The reaction involves the addition of a sulfonyl-stabilized carbanion to a carbonyl compound, followed by elimination to form an alkene. In the initial versions of the reactions, the elimination was done under reductive conditions. More recently, a modified version that avoids this step was developed by Sylvestre Julia (no relation to Marc). The former version is sometimes referred to as the Julia-Lythgoe olefination, whereas the latter is called the Julia-Kocienski olefination. In the reductive variant, the adduct is usually acylated and then treated with a reducing agent, such assodium amalgam
Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. ...
or SmI2. Several reviews of these reactions have been published.
Reaction mechanism
The initial steps are straightforward. The phenyl sulfoneanion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
(2) reacts with an aldehyde to form the alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
(3). The alkoxide is functionalized with R3-X to give the stable intermediate (4). The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed through a vinylic radical species (5). Protonation of the vinylic radical gives the desired product (6).
 The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often. This transformation highly favors formation of the ''E''-alkene.
The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often. This transformation highly favors formation of the ''E''-alkene.
Variations
Modified Julia olefination
 The modified Julia olefination, also known as the one-pot Julia olefination is a modification of the classical Julia olefination. The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the
The modified Julia olefination, also known as the one-pot Julia olefination is a modification of the classical Julia olefination. The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the benzothiazole
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivativ ...
sulfone. The reaction of the benzothiazole sulfone (1) with lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
(LDA) gives a metallated benzothiazolyl sulfone, which reacts quickly with aldehydes (or ketones) to give an alkoxide intermediate (2). Unlike the phenyl sulfones, this alkoxide intermediate (2) is more reactive and will undergo a Smiles rearrangement to give the sulfinate salt (4). The sulfinate salt (4) will spontaneously eliminate sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
and lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
benzothiazolone (5) producing the desired alkene (6).
Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition. As a result, this reaction often generates a mixture of alkene stereoisomers.
Julia–Kocienski olefination
 The Julia–Kocienski Olefination, a further refinement of the Modified Julia olefination, offers very good ''E''-selectivity. In the Julia–Kocienski olefination the
The Julia–Kocienski Olefination, a further refinement of the Modified Julia olefination, offers very good ''E''-selectivity. In the Julia–Kocienski olefination the alkylating agent Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
is a tetrazole
A tetrazole is a chemical synthesis, synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound - a whitish crystalline powder with the f ...
. It proceeds with the same mechanism as the benzothiazole sulfone above. The high ''E''-selectivity of the Julia–Kocienski olefination is the result of kinetically controlled diastereoselective addition of metalated 1-phenyl-1H-tetrazol-5-yl (PT) sulfones to nonconjugated aldehydes. This yields anti-β-alkoxysulfones which stereospecifically decompose to the ''E''-alkenes. In one adaptation, with ''t-butyltetrazoylmethyl sulfone'' the reaction conditions are either sodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula . This species, usually called NaHMDS (sodium hexamethyldisilazide), is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are ...
at −70 °C in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
or caesium carbonate
Caesium carbonate or cesium carbonate is a chemical compound with the chemical formula . It is white crystalline solid. Caesium carbonate has a high solubility in polar solvents such as water, ethanol and DMF. Its solubility is higher in organic ...
at +70 °C. This reaction is named after Philip J. Kocienski for his modification to the Julia olefination.

Synthetic Applications
The Julia or modified Julia olefination reaction is a powerful and versatile synthetic transformation, widely utilized in the construction of complex natural products with excellent control of geometrical isomerism.Pterostilbene
Pterostilbene
Pterostilbene () (trans-3,5-dimethoxy-4-hydroxystilbene) is a stilbenoid chemically related to resveratrol. In plants, it serves a defensive phytoalexin role.
Natural occurrence
Pterostilbene is found in almonds, various ''Vaccinium'' berries ( ...
is a stilbenoid chemically related to resveratrol. It belongs to the group of phytoalexins, agents produced by plants to fight infections. Pterostilbene is a naturally occurring dimethyl ether analog of resveratrol. It is believed that the compound also has anti-diabetic
Drugs used in diabetes treat types of diabetes mellitus by decreasing glucose levels in the blood. With the exception of insulin, most GLP-1 receptor agonists ( liraglutide, exenatide, and others), and pramlintide, all diabetes medications a ...
properties, but so far very little has been studied on this issue.
Compared to the Wittig, Wittig-Horner, Perkin, or transition-metal-catalyzed reactions to synthesize pterostilebene, the Julia olefination offers a simple, economical alternative method for preparation of pterostilbene.

Resveratrol
One adaptation of the Julia-Kocienski olefination gives the synthesis of thestilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with Chalconoid, chalcones. Most stilbenoids ...
resveratrol
Resveratrol (3,5,4′-trihydroxy-''trans''-stilbene) is a stilbenoid, a type of natural phenol or polyphenol and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacterium, ba ...
, a natural compound found in common foods like grapes, wines and nuts. Resveratrol is a biologically important stilbenoid which has been suggested to have many health benefits. The Julia-Kocienski olefination serves as a powerful reaction in the synthesis of resveratrol analogues with 3,5-bis(trifluoromethyl)phenyl sulfones. The following schematic displays the general scheme for synthesizing resveratrol analogues, where R2 is an aryl group.

(−)-Callystatin A
In the asymmetric total synthesis of (−)-callystatin A by Amos Smith, two separate Julia olefinations were used to append two ''E''-alkene moieties. (−)-callystatin A is a member of the leptomycin family of antibiotics. The following schematic displays the Julia-Kocienski olefination used to achieve the precursor to the natural product, as indicated by use of the PT-sulfone.
See also
*Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifie ...
* Johnson–Corey–Chaykovsky reaction
The Johnson–Corey–Chaykovsky reaction (sometimes referred to as the Corey–Chaykovsky reaction or CCR) is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 b ...
* Peterson olefination
* Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
References
# Julia, M.; Paris, J.-M. '' Tetrahedron Lett.'' 1973, ''14'', 4833–4836. () # Kocienski, P. J.; Lythgoe, B.; Ruston, S. '' J. Chem. Soc., Perkin Trans. 1'' 1978, 829. # Keck, G. E.; Savin, K. A.; Weglarz, M. A. '' J. Org. Chem.'' 1995, ''60'', 3194–3204. () # Kocienski, P. J. ''Phosphorus and Sulfur'' 1985, ''24'', 97–127. (Review) # Kelly, S. E. ''Comprehensive Organic Synthesis'' 1991, ''1'', 792–806. (Review) () # Blakemore, P. R. '' J. Chem. Soc., Perkin Trans. 1'' 2002, 2563–2585. () # Baudin, J. B.; Hareau, G.; Julia, S. A.; Ruel, O. '' Tetrahedron Lett.'' 1991, ''32'', 1175. () # Truce, W. E.; Kreider, E. M.; Brand, W. W. '' Org. React.'' 1970, ''18'', 99. (Review) # Paul R. Blakemore, William J. Cole, Philip J. Kocieński, Andrew Morley ''Synlett
''Synlett'' is an international scientific journal for accounts and rapid communications of original contributions of fundamental research in synthetic organic chemistry. The impact factor of this journal is 2.419 (2017). ''Nature'' featured a br ...
'' 1998, 26–28. ()
# Christophe Aïssa '' J. Org. Chem.'' 2006, ''71'', 360–63. ()
# Zajc, B., & Kumar, R. (2010). Synthesis of Fluoroolefins via Julia-Kocienski Olefination. Synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
, 2010(11), 1822–1836.()
# Langcake, P.; Pryce, R. J. (1977). "A new class of phytoalexins from grapevines". Experientia 33 (2): 151–2. () .
# Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D. Heck arylation of styrenes with arenediazonium salts: Short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues. Tetrahedron Lett. 2008, 49(39), 5668–5671.
# Prabhakar Peddikotla, Amar G. Chittiboyina, Ikhlas A. Khan, (2014) ChemInform Abstract: Synthesis of Pterostilbene by Julia Olefination. ChemInform 45, .
# Alonso DA, Fuensanta M, Nájera C, Varea M. J. Org. Chem. 2005; 70:6404–6416. .
# A. B. Smith, III and B. M. Brandt. Total Synthesis of (–)-Callystatin A. Org. Lett. 2001, 3, 1685–1688.
# Robiette, R.; Pospíšil, J. On the Origin of E/Z Selectivity in the Modified Julia Olefination: Importance of the Elimination Step; Eur. J. Org. Chem. 2013, 836–840.
External links
Julia-Lythgoe Olefination
{{Alkenes Olefination reactions Carbon-carbon bond forming reactions Addition reactions Free radical reactions Name reactions