Fluorine Compounds on:
[Wikipedia]
[Google]
[Amazon]

 The
The 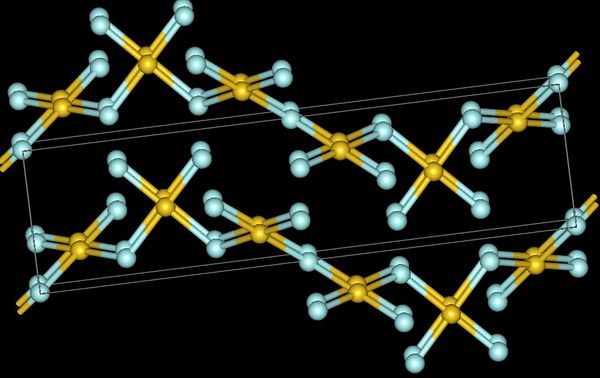 Gold trifluoride adopts a structure of linked –AuF4– squares that align in a helix (spiral chain). In contrast to gold's distinctly ionic trifluoride, its trichloride and tribromide are volatile dimeric molecules. Aluminium trifluoride is a high melting point solid which is a monomer in the gas phase, while its other trihalides are low-melting, volatile molecules or linear polymeric chains that form dimers as gases phase. No trifluoride is soluble in water, but several are soluble in other solvents.
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium, hafnium, plus many of the actinides form tetrafluorides with an ionic structure that puts the metal cation in an 8-coordinate
Gold trifluoride adopts a structure of linked –AuF4– squares that align in a helix (spiral chain). In contrast to gold's distinctly ionic trifluoride, its trichloride and tribromide are volatile dimeric molecules. Aluminium trifluoride is a high melting point solid which is a monomer in the gas phase, while its other trihalides are low-melting, volatile molecules or linear polymeric chains that form dimers as gases phase. No trifluoride is soluble in water, but several are soluble in other solvents.
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium, hafnium, plus many of the actinides form tetrafluorides with an ionic structure that puts the metal cation in an 8-coordinate
 Metal
Metal 

 The
The
 The binary compounds xenon include
The binary compounds xenon include
 When all hydrogens are replaced with fluorine to achieve perfluoroalkanes, a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.
However, if a perfluorocarbon contains double or triple bonds (perfluoro
When all hydrogens are replaced with fluorine to achieve perfluoroalkanes, a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.
However, if a perfluorocarbon contains double or triple bonds (perfluoro

Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
forms a great variety of chemical compounds, within which it always adopts an oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
s, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
(a weaker bridging link to certain nonmetals). Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds.In this article, metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
s are not treated separately from metals and nonmetals, but among elements they are closer to. For example, germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
is treated as a metal, and silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
as a nonmetal. Antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
is included for comparison among nonmetals, even though it is closer to metals chemically than to nonmetals. The noble gases are treated separately from nonmetals; hydrogen is discussed in the Hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
section and carbon in the Organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
section. P-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-bl ...
period 7 element
A period 7 element is one of the chemical elements in the seventh row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the e ...
s have not been studied and thus are not included.
For many elements (but not all) the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others (elements in certain groups) the highest oxidation states of oxides and fluorides are always equal.
Difluorine
While an individual fluorine atom has one unpaired electron, molecular fluorine (F2) has all the electrons paired. This makes itdiamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
(slightly repelled by magnets) with the magnetic susceptibility
In electromagnetism, the magnetic susceptibility (; denoted , chi) is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization (magnetic moment per unit volume) to the applied magnet ...
of −1.2×10−4 ( SI), which is close to theoretical predictions. In contrast, the diatomic molecules of the neighboring element oxygen, with two unpaired electrons per molecule, are paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
(attracted to magnets).
The fluorine–fluorine bond of the difluorine molecule is relatively weak when compared to the bonds of heavier dihalogen molecules. The bond energy is significantly weaker than those of Cl2 or Br2 molecules and similar to the easily cleaved oxygen–oxygen bonds of peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s or nitrogen–nitrogen bonds of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
s. The covalent radius of fluorine of about 71 picometers found in F2 molecules is significantly larger than that in other compounds because of this weak bonding between the two fluorine atoms. This is a result of the relatively large electron and internuclear repulsions, combined with a relatively small overlap of bonding orbitals arising due to the small size of the atoms.
The F2 molecule is commonly described as having exactly one bond (in other words, a bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
of 1) provided by one p electron per atom, as are other halogen X2 molecules. However, the heavier halogens' p electron orbitals partly mix with those of d orbitals, which results in an increased effective bond order; for example, chlorine has a bond order of 1.12. Fluorine's electrons cannot exhibit this d character since there are no such d orbitals close in energy to fluorine's valence orbitals. This also helps explain why bonding in F2 is weaker than in Cl2.
Reactivity
Reactions with elemental fluorine are often sudden or explosive. Many substances that are generally regarded as unreactive, such as powdered steel, glass fragments, andasbestos
Asbestos ( ) is a group of naturally occurring, Toxicity, toxic, carcinogenic and fibrous silicate minerals. There are six types, all of which are composed of long and thin fibrous Crystal habit, crystals, each fibre (particulate with length su ...
fibers, are readily consumed by cold fluorine gas. Wood and even water burn with flames when subjected to a jet of fluorine, without the need for a spark.
Reactions of elemental fluorine with metals require diverse conditions that depend on the metal. Often, the metal (such as aluminium, iron, or copper) must be powdered because many metals passivate by forming protective layers of the metal fluoride that resist further fluoridation. The alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s can react with fluorine explosively, while the alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s react not quite as aggressively. The noble metal
A noble metal is ordinarily regarded as a metallic chemical element, element that is generally resistant to corrosion and is usually found in nature in its native element, raw form. Gold, platinum, and the other platinum group metals (ruthenium ...
s ruthenium, rhodium, palladium, platinum, and gold react least readily, requiring pure fluorine gas at 300–450 °C (575–850 °F).
Fluorine reacts explosively with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
in a manner similar to that of alkali metals. The halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s react readily with fluorine gas as does the heavy noble gas radon
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only Rn has a sufficiently long half-life (3.825 days) for it to b ...
. The lighter noble gases xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
can be made to react with fluorine under special conditions, while argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
will undergo chemical transformations only with hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
. Nitrogen, with its very stable triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s, requires electric discharge and high temperatures to combine with fluorine directly.
Fluorine reacts with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
to form nitrogen and hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
.
Chemical characteristics, effects of presence in a molecule
Fluorine's chemistry is dominated by its strong tendency to gain an electron. It is the mostelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
element and elemental fluorine is a strong oxidant. The removal of an electron from a fluorine atom requires so much energy that no known reagents are known to oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
fluorine to any positive oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
.
Therefore, fluorine's only common oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
is −1. It differs from this value in elemental fluorine, where the atoms are bonded to each other and thus at oxidation state 0, and a few polyatomic ions: the very unstable anions and with intermediate oxidation states exist at very low temperatures, decomposing at around 40 K. (In very rare circumstance, fluorine can exist in zero oxidation state other than elemental form - namely, in AuF7 and in cluster of SF6+ with helium atoms). Also, the cation and a few related species have been predicted to be stable.
Fluorine forms compounds with all elements except neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
and helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
. In particular, it forms binary compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended soli ...
s, named fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typi ...
, with all said elements except argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
. All of the elements up to einsteinium
Einsteinium is a synthetic chemical element; it has symbol Es and atomic number 99 and is a member of the actinide series and the seventh transuranium element.
Einsteinium was discovered as a component of the debris of the first hydrogen bomb ...
, element 99, have been checked except for astatine
Astatine is a chemical element; it has Symbol (chemistry), symbol At and atomic number 85. It is the abundance of elements in Earth's crust, rarest naturally occurring element in the Earth's crust, occurring only as the Decay chain, decay product ...
and francium
Francium is a chemical element; it has symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called '' actinium K'' after the natural decay chain in which it appears), has a half-l ...
, and fluorine is also known to form compounds with mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
, element 101, rutherfordium
Rutherfordium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Rf and atomic number 104. It is named after physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a p ...
, element 104, and seaborgium
Seaborgium is a synthetic chemical element; it has symbol Sg and atomic number 106. It is named after the American nuclear chemist Glenn T. Seaborg. As a synthetic element, it can be created in a laboratory but is not found in nature. It is als ...
, element 106.
As a result of its small size and high negative charge density, the fluoride anion is the "hardest" base (i.e., of low polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of particles with an electric charge. When subject to an elect ...
).
As a part of a molecule, it is a part with great inductive effect
In Organic chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a ...
. In the latter case, it significantly increases the acidity of a molecule: the anion formed after giving the proton off becomes stable as a result. Consider acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
and its mono-
Numeral or number prefixes are prefixes derived from numerals or occasionally other numbers. In English and many other languages, they are used to coin numerous series of words. For example:
* triangle, quadrilateral, pentagon, hexagon, oc ...
, di-, and trifluoroacetic derivatives and their pKa values (4.74, 2.66, 1.24, and 0.23); in other words, the trifluoro derative is 33,800 times stronger an acid than acetic. Fluorine is a principal component of the strongest known charge-neutral acid, fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This mixture is a superacid stronger than pure sulfuric acid, by many orders of magnitude, acco ...
(). There is evidence for an even stronger acid called fluoroauric acid () but it has not proved isolable.
Hydrogen fluoride
Fluorine combines with hydrogen to make a compound (HF) called hydrogen fluoride or, especially in the context of water solutions, hydrofluoric acid. The H-F bond type is one of the few capable of hydrogen bonding (creating extra clustering associations with similar molecules). This influences various peculiar aspects of hydrogen fluoride's properties. In some ways the substance behaves more like water, also very prone to hydrogen bonding, than one of the other hydrogen halides, such as HCl. Hydrogen bonding amongst HF molecules gives rise to highviscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
in the liquid phase and lower than expected pressure in the gas phase. Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides which boil between −85 °C and −35 °C (−120 °F and –30 °F). HF is miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically ...
with water (will dissolve in any proportion), while the other hydrogen halides have large solubility gaps with water. Hydrogen fluoride and water also form several compounds in the solid state, most notably a 1:1 compound that does not melt until −40 °C (−40 °F), which is 44 degrees Celsius (79 degrees Fahrenheit) above the melting point of pure HF.
Unlike other hydrohalic acids, such as hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, hydrogen fluoride is only a weak acid
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation or ionization of a strong acid in solution is effectively complete, except in its most concentrated s ...
in water solution, with acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. I ...
(pKa) equal to 3.19. HF's weakness as an aqueous acid is paradoxical considering how polar
Polar may refer to:
Geography
* Geographical pole, either of the two points on Earth where its axis of rotation intersects its surface
** Polar climate, the climate common in polar regions
** Polar regions of Earth, locations within the polar circ ...
the HF bond is, much more so than the bond in HCl, HBr, or HI. The explanation for the behavior is complicated, having to do with various cluster-forming tendencies of HF, water, and fluoride ion, as well as thermodynamic issues. At great concentrations, a property called homoconjugation is revealed. HF begins to accept fluoride ions, forming the polyatomic ions (such as bifluoride
The bifluoride ion is an inorganic anion with the chemical formula . The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves ...
, ) and protons, thus greatly increasing the acidity of the compound. Hydrofluoric acid is also the strongest of the hydrohalic acids in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
and similar solvents. Its hidden acidity potential is also revealed by the fact it protonates acids like hydrochloric, sulfuric, or nitric. Despite its weakness, hydrofluoric acid is very corrosive, even attacking glass (hydrated only).
Dry hydrogen fluoride dissolves low-valent metal fluorides readily. Several molecular fluorides also dissolve in HF. Many proteins and carbohydrates can be dissolved in dry HF and can be recovered from it. Most non-fluoride inorganic chemicals react with HF rather than dissolving.
Metal fluorides
Metal fluorides are rather dissimilar from other metal halides, adopting distinctive structures. In many respects, metal fluorides are more similar tooxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s, often having similar bonding and crystal structures.
Owing to its high electronegativity, fluorine stabilizes metals in higher oxidation states with high M:halide ratios. Numerous charge-neutral penta- and hexafluorides are known, whereas analogous chlorides and bromides are rarer. The molecular binary fluorides are often volatile, either as solids liquids, or gases at room temperature.
The solubility of fluorides varies greatly but tends to decrease as the charge on the metal ion increases. Dissolved fluorides produce basic solutions.
Low oxidation state metal fluorides
 The
The alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s form monofluorides. All are soluble and have the sodium chloride (rock salt) structure, Because the fluoride anion is basic, many alkali metal fluorides form bifluoride
The bifluoride ion is an inorganic anion with the chemical formula . The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves ...
s with the formula MHF2. Among other monofluorides, only silver(I) and thallium(I) fluorides are well-characterized. Both are very soluble, unlike the other halides of those metals.
Unlike the monofluorides, the difluorides may be either soluble or insoluble. Several transition metal difluorides, such as those of copper(II) and nickel(II), are soluble. The alkaline earth
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are al ...
metals form difluorides that are insoluble. In contrast, the alkaline earth chlorides are readily soluble.
Many of the difluorides adopt the fluorite structure, named after calcium fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble in water. It occurs as the mineral fluorite (also called fluorspar), which is often deeply col ...
(and also adopted by several metal dioxides such as CeO2, UO2, ThO2, etc.), which surrounds each metal cation with 8 fluorides. Some difluorides adopt the rutile structure
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible ...
, named after a form of titanium dioxide and adopted by several other metal dioxides also. The structure is tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
and puts metal atoms in octahedral coordination.
Beryllium difluoride is different from the other difluorides. In general, beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
has a tendency to bond covalently
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
, much more so than the other alkaline earths and its fluoride is partially covalent (although still more ionic than its other halides). BeF2 has many similarities to SiO2 (quartz) a mostly covalently bonded network solid
A network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) is a chemical compound (or element) in which the atoms are bonded by covalent bonds in a continuous network extending throughout the mat ...
. BeF2 has tetrahedrally coordinated metal and forms glasses (is difficult to crystallize). When crystalline, beryllium fluoride has the same room temperature crystal structure as quartz and shares many higher temperatures structures also.
Beryllium difluoride is very soluble in water, unlike the other alkaline earths. (Although they are strongly ionic, they do not dissolve because of the especially strong lattice energy
In chemistry, the lattice energy is the energy change (released) upon formation of one mole of a crystalline compound from its infinitely separated constituents, which are assumed to initially be in the gaseous state at 0 K. It is a measure of ...
of the fluorite structure.) However, BeF2 has much lower electrical conductivity when in solution or when molten than would be expected if it were ionic.
Many metals form trifluorides, such as iron, bismuth, the rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set o ...
s, and the metals in the aluminium and scandium columns of the periodic table. The trifluorides of many rare earths, as well as bismuth, have the YF3 structure. Trifluorides of plutonium, samarium (at high temperature), and lanthanum adopt the LaF3 structure. Iron and gallium trifluorides have the FeF3 structure, which is similar to rhenium trioxide
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements ( Mn, Tc, Re).
Preparat ...
. Only ScF3 is cubic (ReO3) at ambient temperature; this material also has the unusual property of negative thermal expansion
Negative thermal expansion (NTE) is an unusual physicochemical process in which some materials contract upon heating, rather than expand as most other materials do. The most well-known material with NTE is water at 0 to 3.98 °C. Also, the d ...
, meaning it shrinks on heating, over a quite broad temperature range.
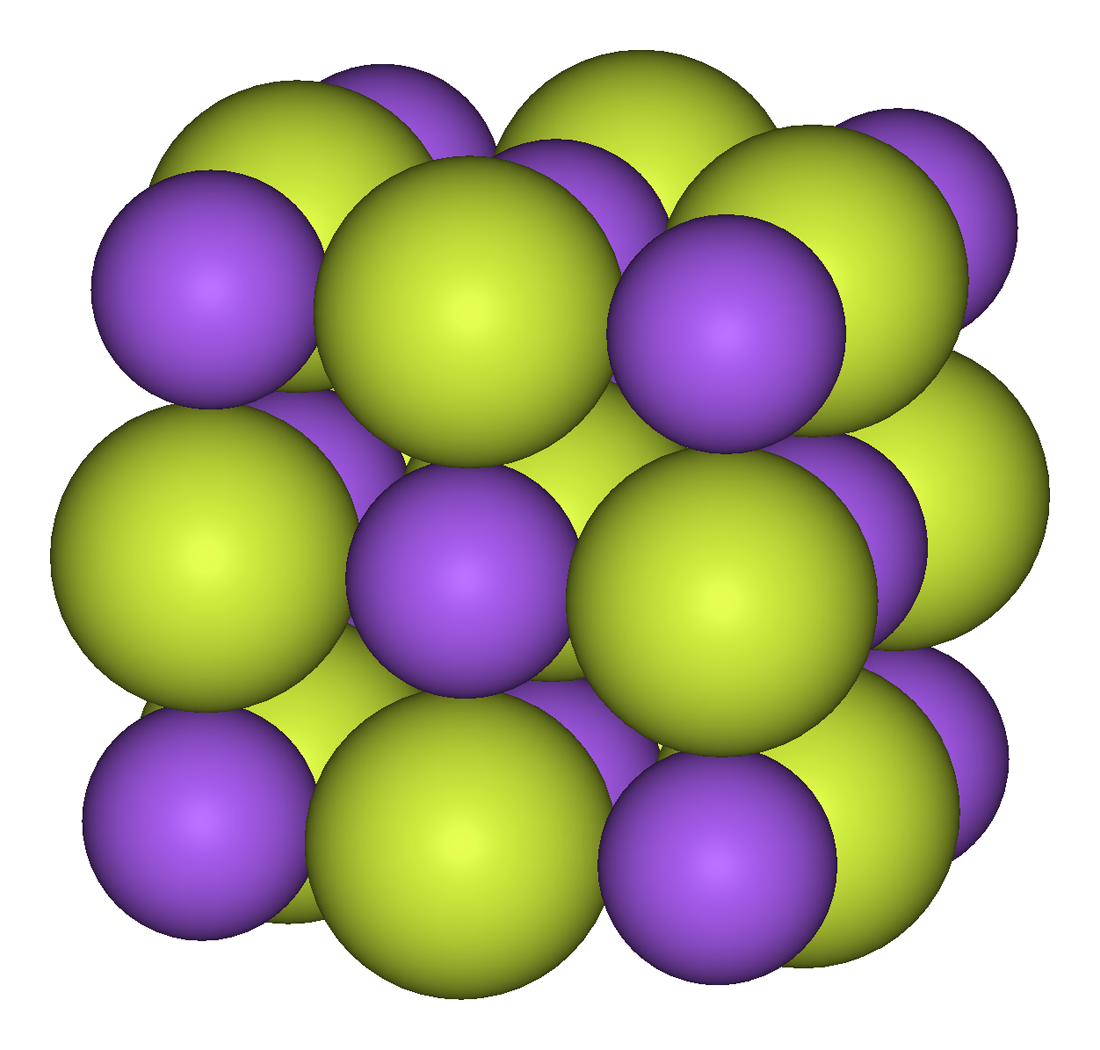
 Gold trifluoride adopts a structure of linked –AuF4– squares that align in a helix (spiral chain). In contrast to gold's distinctly ionic trifluoride, its trichloride and tribromide are volatile dimeric molecules. Aluminium trifluoride is a high melting point solid which is a monomer in the gas phase, while its other trihalides are low-melting, volatile molecules or linear polymeric chains that form dimers as gases phase. No trifluoride is soluble in water, but several are soluble in other solvents.
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium, hafnium, plus many of the actinides form tetrafluorides with an ionic structure that puts the metal cation in an 8-coordinate
Gold trifluoride adopts a structure of linked –AuF4– squares that align in a helix (spiral chain). In contrast to gold's distinctly ionic trifluoride, its trichloride and tribromide are volatile dimeric molecules. Aluminium trifluoride is a high melting point solid which is a monomer in the gas phase, while its other trihalides are low-melting, volatile molecules or linear polymeric chains that form dimers as gases phase. No trifluoride is soluble in water, but several are soluble in other solvents.
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium, hafnium, plus many of the actinides form tetrafluorides with an ionic structure that puts the metal cation in an 8-coordinate square antiprism
In geometry, the square antiprism is the second in an infinite family of antiprisms formed by an even number, even-numbered sequence of triangle sides closed by two polygon caps. It is also known as an ''anticube''.
If all its faces are regular ...
. Melting points are around 1000 °C.
Titanium and tin tetrafluorides are polymeric, with melting points below 400 °C. (In contrast, their tetrachlorides are molecular and liquids at room temperature.) Vanadium tetrafluoride has a similar structure to tin's and disproportionates at 100–120 °C to the trifluoride and the pentafluoride.
The tetrafluorides of iridium, platinum, palladium, and rhodium all share the same structure which was not known until 1975. They have octahedrally coordinated metal atoms with four of the fluorines shared and two unshared. The melting points, where known, are below 300 °C.
Manganese tetrafluoride is an unstable solid that decomposes even at room temperature. Only one of the two allotropes, α-MnF4, is understood. In this compound, manganese forms –MnF6– octahedra which share bridging fluorines to make –Mn4F20– rings which are then further connected three dimensionally.
High oxidation state metal fluorides
Metal penta- and higher fluorides are all molecular and hence at least somewhat volatile.Vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
, niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
, and tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
form pentafluorides as their highest charge-neutral fluoride. Vanadium pentafluoride is the only non-volatile high-charged metal fluoride, with vanadium being centers of –VF6– octahedra. The niobium and tantalum pentafluorides, have the same tetrahedra in their structures, with the difference being the formation of the tetra- (rather than poly-) meric molecules.
Bismuth's highest fluoride is a volatile penta species that is a powerful fluorinating agent. In the solid state, it is polymeric, consisting of linear chains of octahedra, sharing axial fluorides. In combination with alkali metals, pentavalent bismuth can form hexafluorobismuthate, iF6sup>−, upon reaction with a fluoride donor, either strong (such as NaF) or not (such as XeF4).
Many metals that form hexafluoride A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In t ...
s also can form pentafluorides. For instance, uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, which has a well-known hexafluoride A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In t ...
, also forms two different pentafluoride structures. The room-temperature (alpha) form has the same linear chain structure as bismuth pentafluoride. As a molecular (gas) species, UF5 has a square pyramidal structure.
The metals that make well-characterized hexafluoride A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In t ...
s include nine metals in the center of the periodic table (molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
, technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
, ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
, rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
, tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
, iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
, and platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
) along with elements 92–94: uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar Syste ...
, and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
. At room temperature, tungsten hexafluoride
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula . It is a toxic, corrosive, colorless gas, with a density of about (roughly 11 times heavier than air). It is the densest known gas under stand ...
is a gas. Molybdenum hexafluoride and rhenium hexafluoride are liquids. The rest are volatile solids.
 Metal
Metal hexafluoride A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In t ...
s are oxidants because of their tendency to release fluorines: for example, platinum hexafluoride
Platinum hexafluoride is the chemical compound with the formula Pt F6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation sta ...
was the first compound to oxidize molecular oxygen and xenon. Polonium
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tel ...
also forms a hexafluoride, but it is understudied.
Rhenium is the only metal known to bond with seven fluorides, which is the record for number of charged ligands for a charge-neutral metal compound. Rhenium heptafluoride adopts a pentagonal bipyramid molecular geometry. Calculations shows that the currently unknown but perhaps possible iridium heptafluoride (report of synthesis is being prepared), technetium heptafluoride, and osmium heptafluoride will also have this structure.
Osmium octafluoride
Osmium () is a chemical element; it has symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest natura ...
was first reported in 1913, but in 1958 that compound was shown to be actually osmium hexafluoride. A 1993 theoretical study predicted very weak bonds in osmium octafluoride and said that it would be difficult to ever detect experimentally. The study predicted that, if made, OsF8 would have Os–F bonds of two different lengths.

Nonmetal fluorides
Thenonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
binary fluorides are volatile compounds. They show a great difference between period 2 and other fluorides. For instance, period 2 element
A period 2 element is one of the chemical elements in the second row (or Periodic table period, period) of the periodic table, periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) ...
s elements fluorides never exceed the octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 compo ...
in their atoms. (Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
is an exception due to its specific position in the periodic table.) Lower-period elements, however, may form hypervalent molecule
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus ...
s, such as phosphorus pentafluoride or sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attache ...
. The reactivity of such species varies greatly—sulfur hexafluoride is inert, while chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula . It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room ...
is extremely reactive—but there are some trends based on periodic table locations.
Boron trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
is a planar molecule. It has only six electrons around the central boron atom (and thus an incomplete octet), but it readily accepts a Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, forming adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
s with lone-pair-containing molecules or ions such as ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
or another fluoride ion which can donate two more electrons to complete the octet. Boron monofluoride
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, ...
is an unusual molecule with higher than single B-F bond. The bond order has been described as 1.4 (intermediate between a single and double bond). It is isoelectronic with N2.
Silicon tetrafluoride
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 ...
, similar to carbon tetrafluoride
Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (Carbon, CFluorine, F4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be c ...
and germanium tetrafluoride, adopts a molecular tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
structure. SiF4 is stable against heating or electric spark, but reacts with water (even moist air), metals, and alkalis, thus demonstrating weak acidic character. Reactions with organomagnesium compounds, alcohols, amines, and ammonia yield adduction compounds. Fluorosilicic acid, a derivative of SiF4, is a strong acid in aqueous solution (the anhydrous form does not exist).
Pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
s (nitrogen's periodic table column) show very similar trends in reactivity and acidity of the highest fluorides (pentafluorides) and most common ones (trifluorides), with the said property increasing down the group: NF3 is stable against hydrolysis, PF3 hydrolyzes very slowly in moist air, while AsF3 completely hydrolyzes. SbF3 hydrolyzes only partially because of the increasing ionic character of the bond to fluorine. The compounds are weak Lewis bases, with NF3 again being an exception. The pentafluorides of phosphorus and arsenic are much more reactive than their trifluorides; antimony pentafluoride is such a strong acid that it holds the title of the strongest Lewis acid. Nitrogen is not known to form a pentafluoride, although the tetrafluoroammonium
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced ...
cation () features nitrogen in the formal oxidation state of +5. Nitrogen monofluoride
Nitrogen monofluoride (fluoroimidogen) is a metastable species that has been observed in laser studies. It is isoelectronic with O2. Like boron monofluoride, it is an instance of the rare multiply-bonded fluorine atom. It is unstable with respec ...
is a metastable species that has been observed in laser studies. It is isoelectronic with O2 and, unusually, like BF, has a higher bond order than single-bonded fluorine.
 The
The chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
s (oxygen's periodic table column) are somewhat similar: The tetrafluorides of S, Se, and Te hydrolyze and are Lewis acidic. Sulfur and selenium tetrafluorides are molecular while TeF4 is a polymer. The hexafluorides are the result of direct fluorination of the elements (compare: other hexahalides of these elements do not even exist). They increase in reactivity with atomic number: SF6 is extremely inert, SeF6 is less noble (for example, reacts with ammonia at 200 °C (400 °F)), and TeF6 easily hydrolyzes to give an oxoacid. Oxygen's highest fluoride is oxygen difluoride, but fluorine can theoretically (as of 2012) oxidize it to a uniquely high oxidation state of +4 in the fluorocation: . In addition, several chalcogen fluorides occur which have more than one chalcogen (O2F2, S2F10, etc.).
The well-characterized heavier halogens (chlorine, bromine, and iodine) all form mono-, tri-, and pentafluorides: XF, XF3, and XF5. Of the neutral +7 species, only iodine heptafluoride
Iodine heptafluoride is an interhalogen compound with the chemical formula I F7. It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement c ...
is known. While chlorine and bromine heptafluorides are not known, the corresponding cations and , extremely strong oxidizers, are. Astatine is not well-studied, and although there is a report of a non-volatile astatine monofluoride, its existence is debated. Many of the halogen fluorides are powerful fluorinators. Chlorine trifluoride is particularly noteworthy—readily fluorinating asbestos and refractory oxides—and may be even more reactive than chlorine pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula . This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its h ...
. Used industrially, ClF3 requires special precautions similar to those for fluorine gas because of its corrosiveness and hazards to humans.
Superacids
Several important inorganic acids contain fluorine. They are generally very strong because of the high electronegativity of fluorine. One such acid,fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This mixture is a superacid stronger than pure sulfuric acid, by many orders of magnitude, acco ...
(HSbF6), is the strongest charge-neutral acid known. The dispersion of the charge on the anion affects the acidity of the solvated proton (in form of ): The compound has an extremely low pKa of −28 and is 10 quadrillion (1016) times stronger than pure sulfuric acid. Fluoroantimonic acid is so strong that it protonates otherwise inert compounds like hydrocarbons. Hungarian-American chemist George Olah received the 1994 Nobel Prize in chemistry for investigating such reactions.
Noble gas compounds
Thenoble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es are generally non-reactive because they have filled electronic shells. Until the 1960s, no chemical bond with a noble gas was known. In 1962, Neil Bartlett found that platinum hexafluoride
Platinum hexafluoride is the chemical compound with the formula Pt F6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation sta ...
reacts with xenon. He called the compound he prepared xenon hexafluoroplatinate
Xenon hexafluoroplatinate is the product of the reaction of platinum hexafluoride with xenon, in an experiment that proved the chemical reactivity of the noble gases. This experiment was performed by Neil Bartlett at the University of British Co ...
, but since then the product has been revealed to be mixture, perhaps monofluoroxenyl(II) pentafluoroplatinate, eFsup>+ tF5sup>−, monofluoroxenyl(II) undecafluorodiplatinate, eFsup>+ t2F11sup>−, and trifluorodixenyl(II) hexafluoroplatinate, e2F3sup>+ tF6sup>−. Bartlett's fluorination of xenon has been highly praised. Later in 1962, xenon was found to react directly with fluorine to form the di- and tetrafluorides. Since then, other noble gas fluorides have been reported.
 The binary compounds xenon include
The binary compounds xenon include xenon difluoride
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as th ...
, xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
: Xe + 2 →
This reaction is exothermic, rele ...
, and xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All of them are exergonic and stable at normal temperatur ...
. Xenon forms several oxyfluorides, such as xenon oxydifluoride, XeOF2, by hydrolysis of xenon tetrafluoride. Its lighter neighbor, krypton also forms well-characterized compounds, e.g., krypton difluoride
Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances ...
. Krypton tetrafluoride was reported in 1963, but was subsequently shown to be a mistaken identification; the compound seems to be very hard to synthesize now (although even the hexafluoride may exist).
In accordance with the periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
, radon is more reactive toward fluorine. Radon difluoride
Radon difluoride () is a compound of radon, a radioactive noble gas. Radon reacts readily with fluorine to form a solid compound, but this decomposes on attempted vaporization and its exact composition is uncertain. Calculations suggest that it ...
has been claimed. The lighter noble gases (helium through argon) do not form stable binary fluorides.
Highest oxidation states: fluorine versus oxygen
Elements frequently have their highest oxidation state in the form of a binary fluoride. Several elements show their highest oxidation state only in a few compounds, one of which is the fluoride; and some elements' highest known oxidation state is seen exclusively in a fluoride. Forgroups
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
1–5, 13–16 (except nitrogen), the highest oxidation states of oxides and fluorides are always equal. Differences are only seen in chromium, groups 7–10, copper, mercury, and the noble gases. Fluorination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. ...
allows some elements to achieve relatively lowThere is no general line where oxidation states are "relatively low" or "relatively high", they rely on specific elements (and defined only for elements that have highest oxides and fluorides are in different oxidation states); in general, +7 and +8 are high, while +4 and below are low. States +5 and +6 rely on element properties, like atomic radius; for a small nitrogen atom, +5 is "high" here, but for larger palladium and platinum +6 is still "low". highest oxidation states that are otherwise hard to achieve. For example, no binary oxide is known for krypton, but krypton difluoride
Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances ...
is well-studied. At the same time, for some other elements, certain very high oxidation states are known only for the oxygen-based species, not the fluorine-based ones. For the previously mentioned volatile oxides, there are no corresponding hepta- or octafluorides except for rhenium. (For example, ruthenium octafluoride is unlikely to be ever synthesized, while ruthenium tetroxide has even found an industrial use.) The main problem that prevents fluorine from forming the highest states in covalent hepta- and octafluorides is that it is hard to attach such a large number of ligands around a single atom; the number of ligands is halved in analogous oxides. However, octafluoride anions, such as the octafluoroiodate (), octafluorozirconate (), and octafluoroxenate () anions are well-known.
The highest oxidation states may be uncommon to everyday life, or even industrial usage. For example, the synthesis of mercury tetrafluoride, the first compound to achieve an oxidation state above +2 for a group 12 element
Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn). Formerly this group was named ''IIB'' (pronounced as "group two B", as the "I ...
, breaking the filled 5d-shell, again showing the significance of the relativistic effects on the heavy elements, and fueling the debate over whether mercury, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, and zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
are transition metals, occurred at cryogenic temperatures and the compound decomposes at the temperatures of solid nitrogen. More unstable still, a rare cobalt(V) species, the cation, has only been observed in gas phase (with no interactions with other atoms, thus no shown stability in any chemical environment). The reason why such unstable species exist is complicated, yet can be summarized as follows on the example of the hypothesized molecule: According to the modern calculations, five fluorine atoms and one nitrogen atoms can theoretically arrange themselves in different ways, such as and , and , , etc. The + system is of the smallest energy (most stable). However, if a molecule was synthesized, it would have to go through a high-energy transitional state, from which it could decay into two molecules. But since the transitional state is higher in energy than the hexatomic molecule, the energy difference would need to be added to reach the transitional state and thus allow the decay. This energy is called reaction activation barrier. (The second decay mode has an analogical position.) Thus if little energy was added (low temperatures), then the compound could exist; however, the synthesis is a serious problem (not yet solved).
Fluorine oxoacids
Fluorine is very difficult to form oxoacids with, due to the fact that it will take a lot of energy per mole to join each additional oxygen atom to fluorine owing to its exceptional electronegativity. The only known oxoacid of fluorine ishypofluorous acid
Hypofluorous acid, chemical formula , is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in this acid (and in t ...
(). It appears as a white-solid below temperatures of -117°C, and a pale-yellow liquid above that temperature. HOF is itself only stable until 0 °C and rapidly decomposes above temperatures above that, being explosive at room temperature. Despite being often found as a short-lasting intermediate in the oxidation of water by fluorine, HOF can be still be stably isolated as a solid and the only hypohalous acid known to be able to do so. Its conjugate base, the hypofluorite anion (), can form certain stable compounds, like lithium hypofluorite
Lithium hypofluorite is an inorganic compound with the chemical formula of . It is a compound of lithium, fluorine, and oxygen. This is a lithium salt of hypofluorous acid, and contains lithium cations and hypofluorite anions .
Synthesis
The s ...
() and trifluoromethyl hypofluorite ().
''Fluorous acid'' (), ''fluoric acid'' (), and '' perfluoric acid'' () are determined to be much, much more unstable than hypofluorous acid and have yet to be discovered.
Organofluorine compounds
The carbon–fluorine chemical bond of theorganofluorine compound
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, ...
s is the strongest bond in organic chemistry. Along with the low polarizability of the molecules, these are the most important factors contributing to the great stability of the organofluorines.
The carbon–fluorine bond of the smaller molecules is formed in three principal ways: Fluorine replaces a halogen or hydrogen, or adds across a multiple bond. The direct reaction of hydrocarbons with fluorine gas can be dangerously reactive, so the temperature may need to be lowered even to −150 °C (−240 °F). "Solid fluorine carriers", compounds that can release fluorine upon heating, notably cobalt trifluoride, may be used instead, or hydrogen fluoride. After the reaction, the molecular size is not changed significantly, as the elements have very similar van der Waals radii. Direct fluorination becomes even less important when it comes to organohalogens or unsaturated compounds reactions, or when a perfluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are ...
is desired (then HF-based electrolysis is typically used). In contrast, the fluoropolymers are formed by polymerizing free radicals; other techniques used for hydrocarbon polymers do not work in that way with fluorine.
The range of organofluorine compounds is diverse, reflecting the inherent complexity of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. A vast number of small molecules exist with varying amounts of fluorine substitution, as well as many polymers—research into particular areas is driven by the commercial value of applications.
Small molecules
Monofluoroalkanes (alkanes with one hydrogen replaced with fluorine) may be chemically and thermally unstable, yet are soluble in many solvents; but as more fluorines are in instead of hydrogens, the stability increases, while melting and boiling points, and solubility decrease. While the densities and viscosities are increased, the dielectric constants, surface tensions, and refractive indices fall. Partially fluorinated alkanes are thehydrofluorocarbon
Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condit ...
s (HFCs). Substituting other halogens in combination with fluorine gives rise to chlorofluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are ...
s (CFCs) or bromofluorocarbons (BFCs) and the like (if some hydrogen is retained, HCFCs and the like). Properties depend on the number and identity of the halogen atoms. In general, the boiling points are even more elevated by combination of halogen atoms because the varying size and charge of different halogens allows more intermolecular attractions. As with fluorocarbons, chlorofluorocarbons and bromofluorocarbons are not flammable: they do not have carbon–hydrogen bond
In chemistry, the carbon–hydrogen bond ( bond) is a chemical bond between carbon and hydrogen atoms that can be found in many organic compounds. This bond is a covalent bond, covalent, single bond, meaning that carbon shares its outer valence el ...
s to react and released halides quench flames.
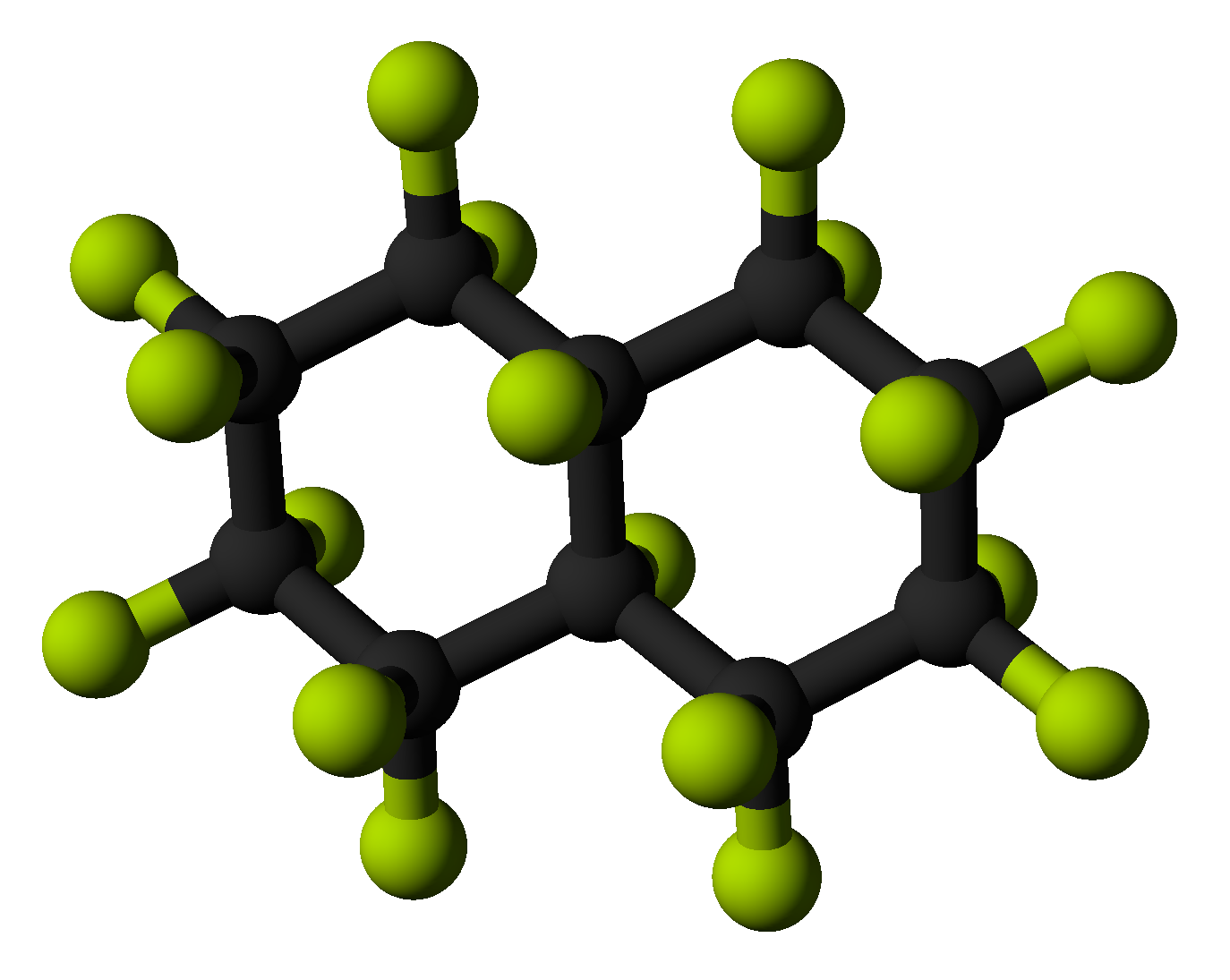
 When all hydrogens are replaced with fluorine to achieve perfluoroalkanes, a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.
However, if a perfluorocarbon contains double or triple bonds (perfluoro
When all hydrogens are replaced with fluorine to achieve perfluoroalkanes, a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.
However, if a perfluorocarbon contains double or triple bonds (perfluoroalkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
or -alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
), a very reactive towards ligand accepting result, even less stable than corresponding hydrocarbons. Difluoroacetylene, which decomposes even under liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
temperatures, is a notable example. If such a molecule is asymmetric, then the more fluorinated carbon is attacked, as it holds positive charge caused by the C–F bonds and is shielded weakly (similarly to that how unsaturated hydrocarbons attacked by HF add hydrogen to the more hydrogen-rich atom per Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
).
Perfluorinated compound
A perfluorinated compound (PFC) or perfluoro compound is an Organofluorine chemistry, organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common ...
s, as opposed to perfluorocarbons, is the term used for molecules that would be perfluorocarbons—only carbon and fluorine atoms—except for having an extra functional group (even though another definition exists). They share most of perfluorocarbon properties (inertness, stability, non-wettingness and insolubility in water and oils, slipperiness, etc.), but may differ because of the functional group properties, although the perfluorocarbon tail differ the group-specific properties as compared to those of hydrocarbon-tailed compounds.
The perfluoroalkanesulfonic acids are also very notable for their acidity. The sulfonic acid derivative, trifluoromethanesulfonic acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for este ...
, is comparable in strength to perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. It is an oxoacid of chlorine. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxid ...
. These compounds lower surface energy; for this reason, they, especially perfluorooctanesulfonic acid
Perfluorooctanesulfonic acid (PFOS) (conjugate acid, conjugate base perfluorooctanesulfonate) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group, and thus it is a perfluorosulfonic acid and a Per ...
(PFOS, formerly the active component in brand "Scotchgard") have found industrial use as surfactants.
If a perfluorinated compound has a fluorinated tail, but also a few non-fluorinated carbons (typically two) near the functional group, it is called a fluorotelomer
Fluorotelomers are fluorocarbon-based oligomers, or telomers, synthesized by telomerization. Some fluorotelomers and fluorotelomer-based compounds are a source of environmentally persistent perfluorinated carboxylic acids such as PFOA and PFNA, w ...
(such molecules are commercially treated as perfluorinated), but such molecules are more of industrial value than chemical. The chain end may similarly be attached to different functional groups (via the hydrogenized terminal carbon), such as hydroxyl resulting in fluorotelomer alcohols, sulfonate resulting in fluorotelomer sulfonates, etc.
Polymers
Fluoropolymers are similar in many regards with smaller molecules; adding fluorine to a polymer affects the properties in the same manner as in small molecules (increasing chemical stability, melting point, reducing flammability, solubility, etc.). Each fluoropolymer has own characteristic properties, though. The simplest fluoroplastic is polytetrafluoroethylene (PTFE, DuPont brand Teflon), which is a simple linear chain polymer with the repeatingstructural unit
In polymer chemistry, a structural unit is a building block of a polymer chain. It is the result of a monomer which has been polymerized into a long chain.
There may be more than one structural unit in the repeat unit. When different monomers ar ...
:–CF2–. PTFE has a backbone of carbons single bonded in a long chain, with all side bonds to fluorines. It contains no hydrogens and can be thought of as the perfluoro analog of polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
(structural unit: –CH2–). PTFE has high chemical and thermal stability, as expected for a perfluorocarbon, much stronger than polyethylene. Its resistance to van der Waals forces
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical ele ...
makes PTFE the only known surface to which a gecko
Geckos are small, mostly carnivorous lizards that have a wide distribution, found on every continent except Antarctica. Belonging to the infraorder Gekkota, geckos are found in warm climates. They range from .
Geckos are unique among lizards ...
cannot stick. The compound, however, lacks an ability to transform upon melting, which is not a problem for various PTFE derivatives, namely FEP (fluorinated ethylene propylene
Fluorinated ethylene propylene (FEP) is a copolymer of hexafluoropropylene and tetrafluoroethylene. It differs from the polytetrafluoroethylene (PTFE) resins in that it is melt-processable using conventional injection molding and Plastic extrusio ...
, with some fluorines replaced with the–CF3 group) or PFA (perfluoroalkoxy
Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). The properties of these polymers are similar to ...
, some fluorines replaced with –OCF3). They share most properties with PTFE, but there are still differences, namely maximum usage temperature (highest for the non-flexible PTFE).
There are other fluoroplastics other than perfluorinated. Polyvinylidene fluoride
Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride. Its chemical formula is (C2H2F2)''n''.
PVDF is a specialty plastic use ...
(PVDF, structural unit: –CF2CH2–), is an analog of PTFE with half the fluorines. PVF (polyvinyl fluoride
Polyvinyl fluoride (PVF) or –(CH2CHF)n– is a polymer material mainly used in the flammability-lowering coatings of airplane interiors and photovoltaic module backsheets. It is also used in raincoats and metal sheeting. Polyvinyl fluoride is a ...
, structural unit: –CH2CHF–) contains one one-fourth the fluorines of PTFE. Despite this, it still has many properties of more fluorinated compounds. PCTFE (polychlorotrifluoroethylene
Polychlorotrifluoroethylene (PCTFE or PTFCE) is a thermoplastic chlorofluoropolymer with the molecular formula , where ''n'' is the number of monomer units in the polymer molecule. It is similar to polytetrafluoroethene (PTFE), except that it is ...
, structural unit: –CF2CFCl–) is another important compound. It differs from PTFE by having a quarter of fluorine replaced with chlorines, yet this difference brings even greater hardness, creep resistance, and moisture persistence.
Mild fluorination of polyethylene gives does not make all of the plastic lose its hydrogens for fluorine; only a thin layer (0.01 mm at maximum) is then affected. This is somewhat similar to metal passivation: the bulk properties are not affected, but the surface properties are, most notably, a greater vapor barrier
A vapor barrier (or vapour barrier) is any material used for damp proofing, typically a plastic or foil sheet, that resists diffusion of moisture through the wall, floor, ceiling, or roof assemblies of buildings and of packaging to prevent inter ...
. Therefore, they are a cheaper alternative to the perfluoro plastics if only surface is important.
Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware (U.S. Patent 3,282,875). Additional work on the polym ...
is a structurally complicated polymer. It has a PTFE-like backbone, but also contains side chains of perfluoro ether that end in sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
(–SO2OH) groups. It also possesses great chemical stability, while exact properties vary with morphology. However, because of the difficult chemical structure, it is also relatively easily converted to an ionomer
An ionomer () ('' iono-'' + '' -mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole perce ...
(shows conductivity) by adding cations like Na+ or by converting into the sulfonic acid rather than the given sulfonyl fluoride. The conductivity is due to that the main carbon chain separates from the side chains, thus forming polar and non-polar regions. This form is also very hydroscopic.
Fluoroelastomer
A fluoroelastomer is a fluorocarbon-based synthetic rubber. Fluoroelastomers generally have wide chemical resistance.
Composition
Several compositions of fluoroelastomers exist including FKM (by ASTM D1418 standard, equivalent to FPM by ISO/DIN ...
s, like other elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s (artificial rubbers), consist of disordered polymer chains connected in three dimensions. The main challenges in making fluorelastomers are cross-linking (reacting the unreactive polymers), as well as removing the HF formed during curing. There are three main families of fluoroelasters. VDF/HFP is a copolymer system of vinylidene fluoride and (at least 20%) hexafluoropropylene. TFE/propylene is another copylymer system with better chemical resistance to some solvents. TFE/PMVE (perfluoromethylvinyl ether) is a copolymer system which creates a perfluorinated fluoroelastomer.
See also
*Fluorochemical industry
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the ...
Notes
Citations
Indexed references
* * * * * * * * ** ** ** * * * {{Chemical compounds by element F