dehydrochlorination on:
[Wikipedia]
[Google]
[Amazon]
 In
In
Dehydrohalogenation of Alkyl Halides
{{Authority control Elimination reactions Olefination reactions
 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, dehydrohalogenation is an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
which removes a hydrogen halide
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, astatine, or ...
from a substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
. The reaction is usually associated with the synthesis of alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s, but it has wider applications.
Dehydrohalogenation from alkyl halides
Traditionally, alkylhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus halides having no C–H bond on an adjacent carbon are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent a ...
dehydrohalogenates to give phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
via a benzyne intermediate.
Base-promoted reactions to alkenes
When treated with a strong base many alkyl chlorides convert to corresponding alkene. It is also called a β-elimination reaction and is a type ofelimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
. Some prototypes are shown below:
:
Here ethyl chloride
Chloroethane, commonly known as ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet ...
reacts with potassium hydroxide, typically in a solvent such as ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, giving ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
. Likewise, 1-chloropropane and 2-chloropropane give propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like od ...
.
Zaitsev's rule
In organic chemistry, Zaytsev's rule (or Zaitsev's rule, Saytzeff's rule, Saytzev's rule) is an 68–95–99.7 rule, empirical rule for predicting the favored alkene product(s) in elimination reactions. While at the University of Kazan, Russian che ...
helps to predict regioselectivity
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
for this reaction type.
In general, the reaction of a haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
with potassium hydroxide can compete with an SN2 nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
reaction by OH− a strong, unhindered nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. Alcohols are however generally minor products. Dehydrohalogenations often employ strong bases such as potassium ''tert''-butoxide (K+ H3sub>3CO−).
Base-promoted reactions to alkynes
Upon treatment with strong base, vicinaldihalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
s convert to alkynes.
Thermal cracking
On an industrial scale, base-promoted dehydrohalogenations as described above are disfavored. The disposal of the alkali halide salt is problematic. Instead thermally-induced dehydrohalogenations are preferred. One example is provided by the production ofvinyl chloride
Vinyl chloride is an organochloride with the formula H2C =CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a ...
by heating 1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl ...
:M. Rossberg et al. "Chlorinated Hydrocarbons" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2006, Wiley-VCH, Weinheim.
:CH2Cl-CH2Cl → CH2=CHCl + HCl
The resulting HCl can be reused in oxychlorination In chemistry, oxychlorination is a process for generating the equivalent of chlorine gas (Cl2) from hydrogen chloride and oxygen. This process is attractive industrially because hydrogen chloride is less expensive than chlorine.
Mechanism
The re ...
reaction.
Thermally induced dehydrofluorinations are employed in the production of fluoroolefins and hydrofluoroolefin
Hydrofluoroolefins (HFOs) are Saturated and unsaturated compounds, unsaturated organic compounds composed of hydrogen, fluorine and carbon. These organofluorine compounds are of interest as refrigerants. Unlike traditional hydrofluorocarbons (HFCs ...
s. One example is the preparation of 1,2,3,3,3-pentafluoropropene
1,2,3,3,3-Pentafluoropropene is the unsaturated fluorocarbon with the formula HFC=C(F)CF. This colorless gas is of interest as a precursor to hydrofluoroolefins (HFOs), which are used as refrigerants in air conditioners. Of the methods reported ...
from 1,1,2,3,3,3-hexafluoropropane:
:CF2HCH(F)CF3 → CHF=C(F)CF3 + HF
Other dehydrohalogenations
Epoxides
Chlorohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol ...
s, compounds with the connectivity R(HO)CH-CH(Cl)R', undergo dehydrochlorination to give epoxides. This reaction is employed industrially to produce millions of tons of propylene oxide
Propylene oxide is an epoxide with the molecular formula C3H6O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols f ...
annually from propylene chlorohydrin:
:CH3CH(OH)CH2Cl + KOH → CH3CH(O)CH2 + H2O + KCl
Isocyanides
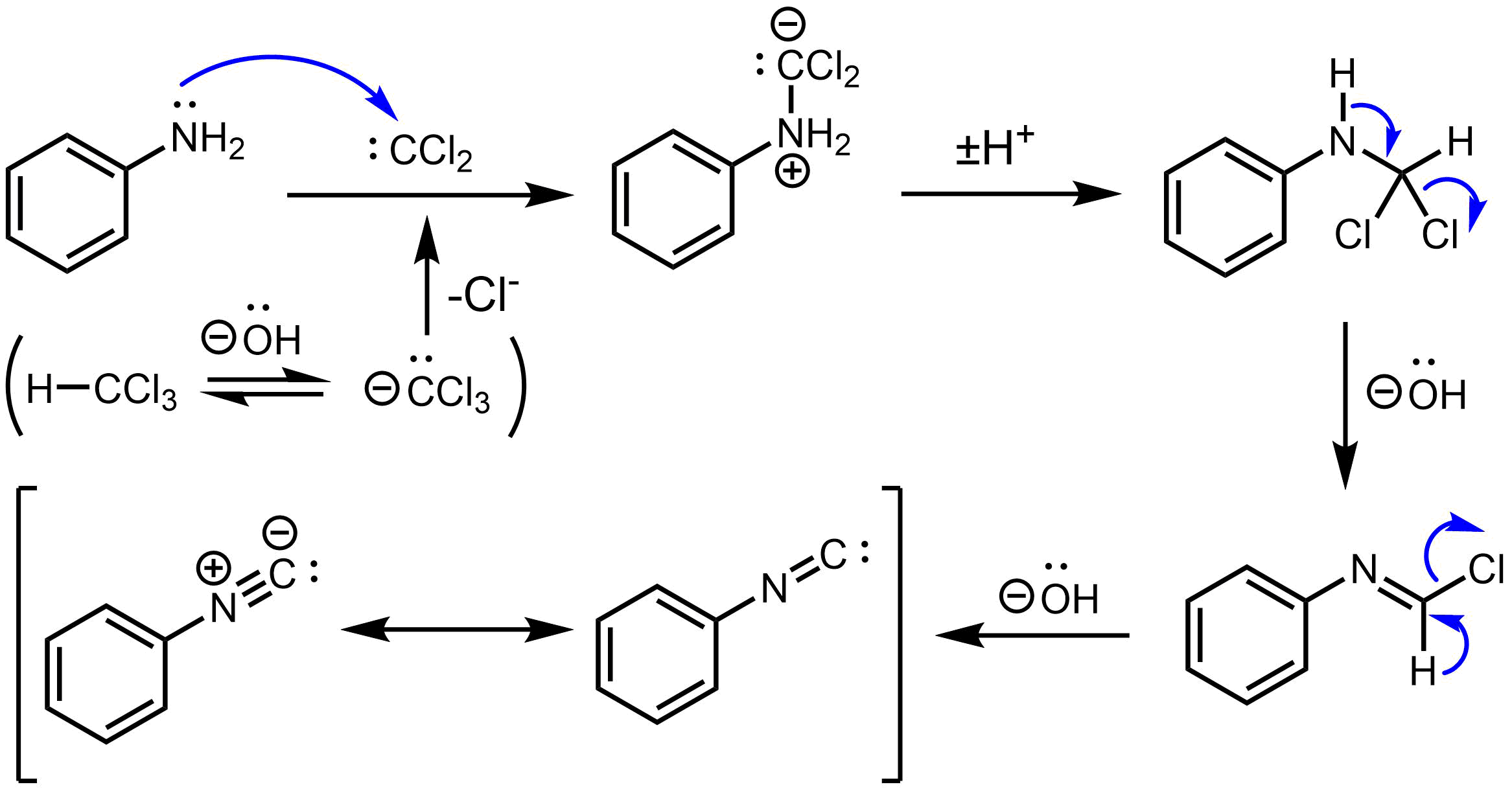
Thecarbylamine reaction
The carbylamine reaction (also known as the Hoffmann isocyanide synthesis) is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene.
Illustrative is ...
for the synthesis of isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
s from the action of chloroform on a primary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
involves three dehydrohalogenations. The first dehydrohalogenation is the formation of dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapi ...
:
:KOH + CHCl3 → KCl + H2O + CCl2
Two successive base-mediated dehydrochlorination steps result in formation of the isocyanide.

Coordination compounds
Dehydrohalogenation is not limited to organic chemistry. Some metal-organiccoordination compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
s can eliminate hydrogen halides, either spontaneously, thermally, or by mechanochemical reaction with a solid base such as potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
.
For example, salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
that contain acidic cations hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
ed to halometallate anions will often undergo dehydrohalogenation reactions reversibly:
: 'B''–Hsup>+··· –ML''n''sup>− ⇌ 'B''–ML''n''+ HX
where ''B'' is a basic ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
such as a pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, X is a halogen (typically chlorine or bromine), M is a metal such as cobalt, copper, zinc, palladium or platinum, and L''n'' are spectator ligands.
References
External links
Dehydrohalogenation of Alkyl Halides
{{Authority control Elimination reactions Olefination reactions