|
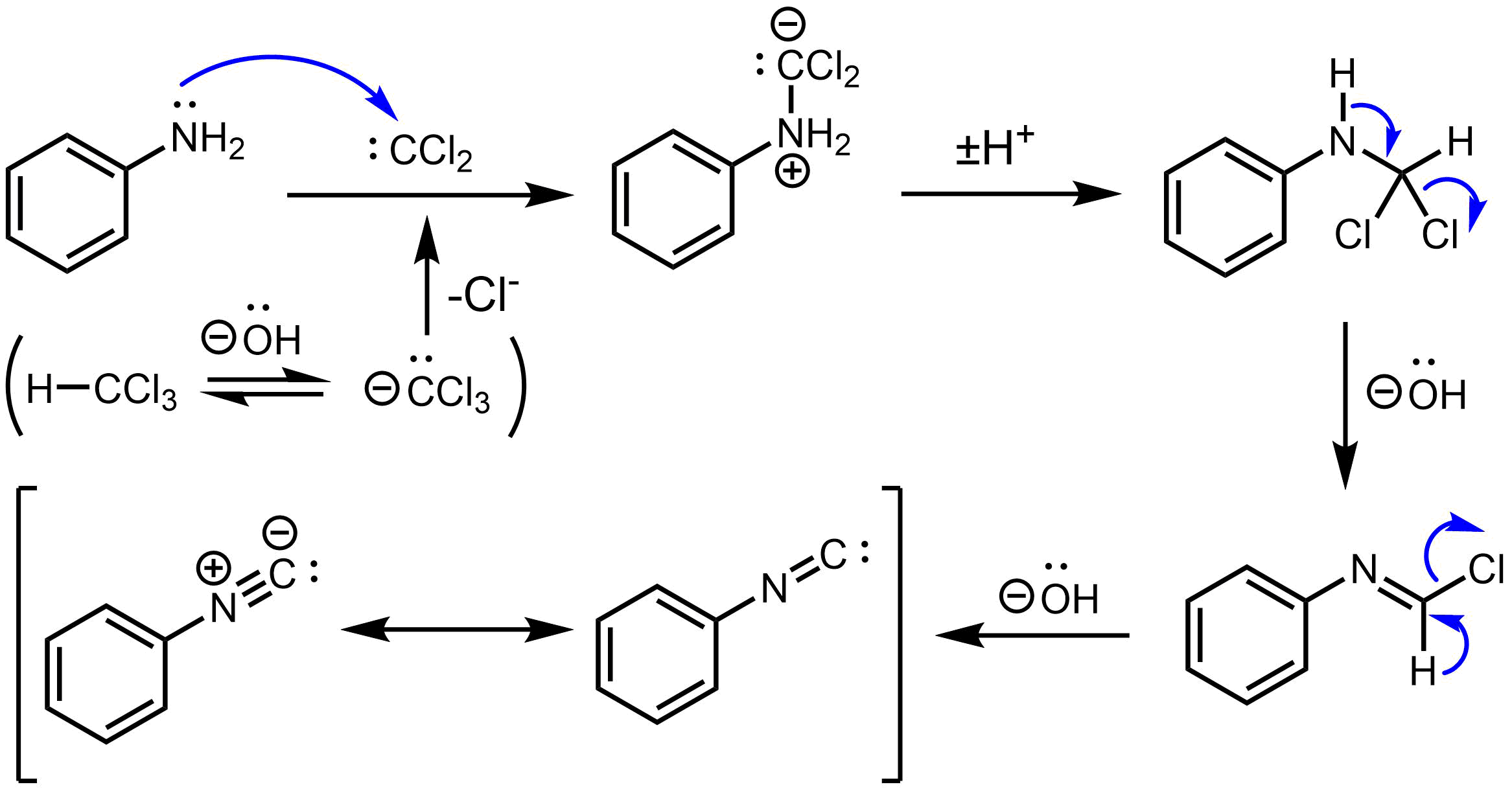
Carbylamine Reaction
The carbylamine reaction (also known as the Hoffmann isocyanide synthesis) is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene. Illustrative is the synthesis of ''tert''-butyl isocyanide from ''tert''-butylamine in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride. :Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O Similar reactions have been reported for aniline. It is used to prepare secondary amines. Test for primary amines As it is only effective for primary amines, the carbylamine reaction can be used as a chemical test for their presence. In this context, the reaction is also known as Saytzeff's isocyanide test. In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide An isocyanide (also called isonitrile or carbylamine) is an organic co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and polytetrafluoroethylene (PTFE). Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C). Structure and name The molecule adopts a tetrahedral molecular geometry with C3v symmetry. The chloroform molecule can be viewed as a methane molecule with three hydrogen atoms replaced with three chlorine atoms, leaving a single hydrogen atom. The name "chloroform" is a portmanteau of ''terchloride'' (tertiary chloride, a trichloride) and ''formyle'', an obsolete name for the methylylidene radical (CH) derived from formic acid. Natural occurrence Many kinds of seaweed produce chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds. Preparation Dichlorocarbene is most commonly generated by reaction of chloroform and a base such as potassium tert-butoxide, potassium ''tert''-butoxide or aqueous sodium hydroxide. A phase transfer catalyst, for instance quaternary ammonium cation, benzyltriethylammonium bromide, facilitates the migration of the hydroxide in the organic phase. :HCCl3 + NaOH → CCl2 + NaCl + H2O Other reagents and routes Another precursor to dichlorocarbene is ethyl trichloroacetate. Upon treatment with sodium methoxide it releases CCl2. Phenyl(trichloromethyl)mercury decomposes thermally to release CCl2. :PhHgCCl3 → CCl2 + PhHgCl Dichlorodiazirine, which is stable in the dark, chemical decomposition, decomposes into dic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butyl Isocyanide
''tert''-Butyl isocyanide is an organic compound with the formula Me3CNC (Me = methyl, CH3). It is an isocyanide, commonly called isonitrile or carbylamine, as defined by the functional group C≡N-R. ''tert''-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor. It forms stable complexes with transition metals and can insert into metal-carbon bonds. ''tert''-Butyl isocyanide is prepared by a Hofmann carbylamine reaction. In this conversion, a dichloromethane solution of ''tert''-butylamine is treated with chloroform and aqueous sodium hydroxide in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride. :Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O ''tert''-Butyl isocyanide is isomeric with pivalonitrile, also known as ''tert''-butyl cyanide. The difference, as with all carbylamine analogs of nitriles, is that the bond joining the CN functional group to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butylamine
''tert''-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. ''tert''-Butylamine is one of the four isomeric amines of butane, the others being ''n''-butylamine, ''sec''-butylamine and isobutylamine. Preparation ''tert''-Butylamine is produced commercially by direct amination of isobutylene using zeolite catalysts: :NH3 + CH2=C(CH3)2 → H2NC(CH3)3 The Ritter reaction of isobutene with hydrogen cyanide is not useful because it produces too much waste. :(CH3)2C=CH2 + HCN + H2O → (CH3)3CNHCHO :(CH3)3CNHCHO + H2O → (CH3)3CNH2 + HCO2H In the laboratory, it can be prepared by the hydrogenolysis of 2,2-dimethylethylenimine, or via ''tert''-butylphthalimide. Uses ''tert''-Butylamine is used as an intermediate in the preparation of the sulfenamides such as ''N''-''tert''-butyl-2-benzothiazylsulfenamide and ''N''-''tert''-butyl-2-benzothiazylsulfeni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form reaction intermediate, intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous catalysis, homogeneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transfer Catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst. PTC is widely exploited industrially.Marc Halpern "Phase-Transfer Catalysis" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Polyesters for example are prepared from acyl chlorides and bisphenol-A. Phosphothioate-based pesticides are generated by PTC-catalyzed alkylation of phosphothioates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Test
In chemistry, a chemical test is a qualitative property, qualitative or Quantitative property, quantitative procedure designed to identify, quantify, or characterise a chemical compound or substituent, chemical group. Purposes Chemical testing might have a variety of purposes, such as to: * Determine if, or verify that, the requirements of a specification, regulation, or contract are met * Decide if a new product development program is on track: Demonstrate proof of concept * Demonstrate the utility of a proposed patent * Determine the interactions of a sample with other known substances * Determine the composition of a sample * Provide Technical standard, standard data for other scientific, medical, and Quality assurance functions * Validate suitability for end-use * Provide a basis for Technical communication * Provide a technical means of comparison of several options * Provide evidence in legal proceedings Biochemical tests * Clinistrips quantitatively test for sugar in uri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utilize its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two Resonance (chemistry), resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrohalogenation
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate (chemistry), substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications. Dehydrohalogenation from alkyl halides Traditionally, alkyl halides are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus halides having no C–H bond on an adjacent carbon are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene dehydrohalogenates to give phenol#Hydrolysis of chlorobenzene, phenol via a benzyne intermediate. Base-promoted reactions to alkenes When treated with a strong base many alkyl chlorides convert to corresponding alkene. It is also called a β-elimination reaction and is a type of elimination reaction. Some prototypes are shown below: :\begin \ce\ &\ce \\ \ce\ &\ce \\ \ce\ &\ce \end Here ethyl chloride reacts with potassium hydroxide, ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |