Coumarone on:
[Wikipedia]
[Google]
[Amazon]
Benzofuran is the
 *
*
heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
consisting of fused benzene and furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol.Laboratory methods
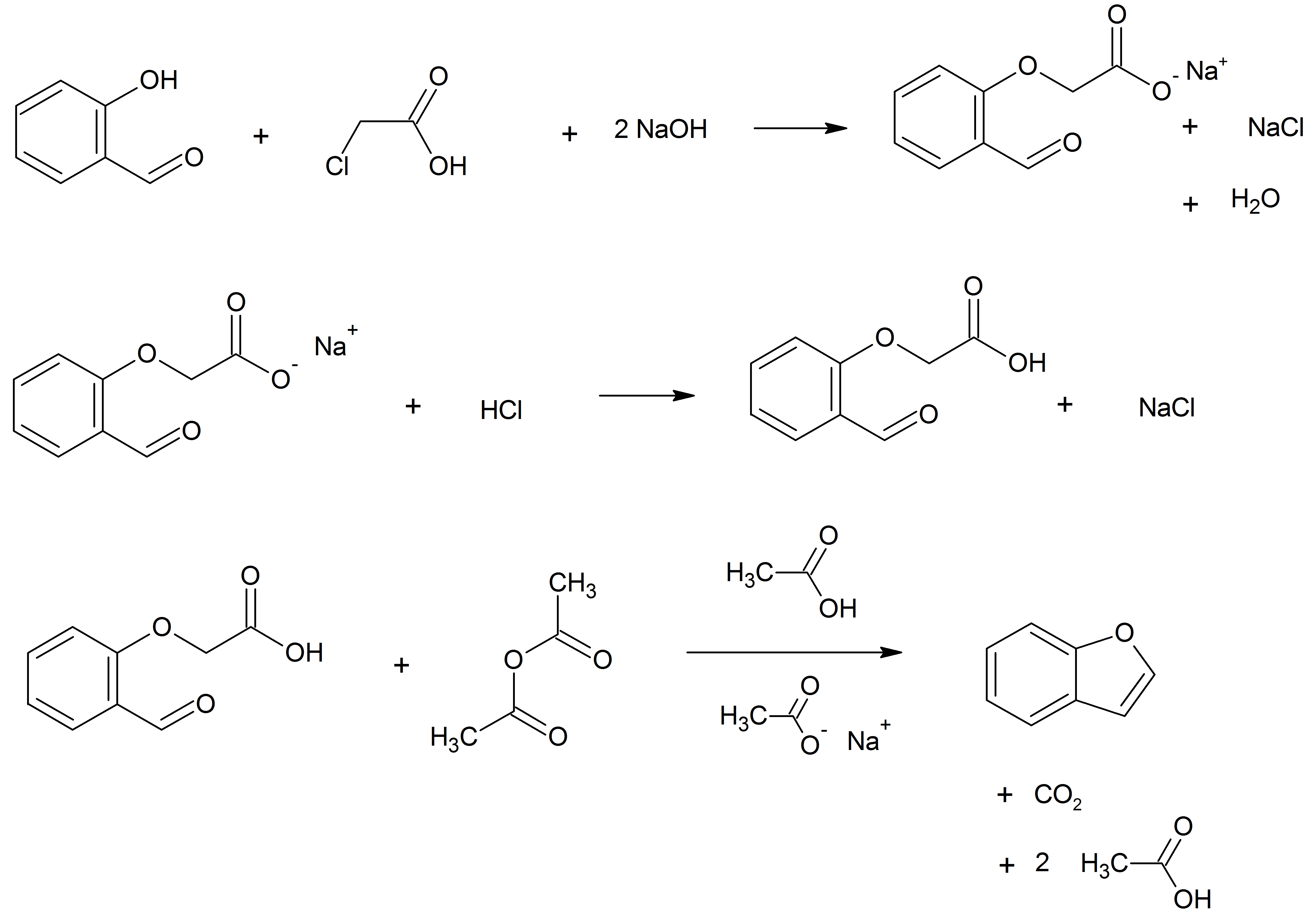
Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *
*Perkin rearrangement
In organic chemistry, the Perkin rearrangement (coumarin–benzofuran ring contraction) is a rearrangement reaction in which a 2-halocoumarin in the presence of hydroxide undergoes a ring contraction to form a benzofuran. The name reaction recogni ...
, where a coumarin is reacted with a hydroxide:
:
*Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
of nitro vinyl furans with various dienophiles:
: Diels–Alder reaction yielding a substituted benzofuran, 450px
*Cycloisomerization Cycloisomerization is any isomerization in which the cyclic compound, cyclic isomer of the substrate is produced in the reaction coordinate. The greatest advantage of cycloisomerization reactions is its atom economy, atom economical nature, by desig ...
of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
ortho-substituted
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
''Ortho'', ''meta'', and ''para'' substitution
...
phenols:
: Benzofurans via Cycloisomerization, 400px
Related compounds
*Substituted benzofuran
The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compou ...
s
* Dibenzofuran, an analog with a second fused benzene ring.
* Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
, an analog without the fused benzene ring.
* Indole, an analog with a nitrogen instead of the oxygen atom.
* Benzothiophene, an analog with a sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
instead of the oxygen atom.
* Isobenzofuran, the isomer with oxygen in the adjacent position.
* Aurone
* Thunberginol F
References
{{Authority control IARC Group 2B carcinogens Simple aromatic rings