|
Benzothiophene
Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo hiophene, a second isomer is known: benzo hiophene. Benzothiophene finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as raloxifene, zileuton, and sertaconazole, and also BTCP. It is also used in the manufacturing of dyes such as thioindigo. Synthesis Most syntheses of benzothiophene create substituted benzothiophenes as a precursor to further reactions. An example is the reaction of an alkyne-substituted 2-bromobenzene with either sodium sulphide or potassium sulphide to form benzothiophene with an alkyl substitution at position 2. Thiourea Thiourea () is an organosulfur c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sertaconazole
Sertaconazole, sold under the brand name Ertaczo among others, is an antifungal medication of the Benzothiophene class. It is available as a cream to treat skin infections such as athlete's foot. It is also available in a vaginal tablet form. The most popular of these is Gyno-Dermofix. Medical uses In randomized, double-blind, multicentre trials of 3 to 6 weeks' duration (n=127-383), a significantly greater number of patients with tinea of the glabrous skin and tinea pedis receiving a topical 2% sertaconazole cream once or twice daily achieved a successful mycological cure compared with recipients of a placebo cream. Side effects Side effects were rarely reported with sertaconazole therapy, but may include contact dermatitis, burning on application site and skin dryness. Pharmacology Pharmacodynamics Sertaconazole has several known mechanisms of action. It is considered fungistatic, fungicidal, antibacterial, antiinflammatory, antitrichomonal, and antipruritic. Like other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
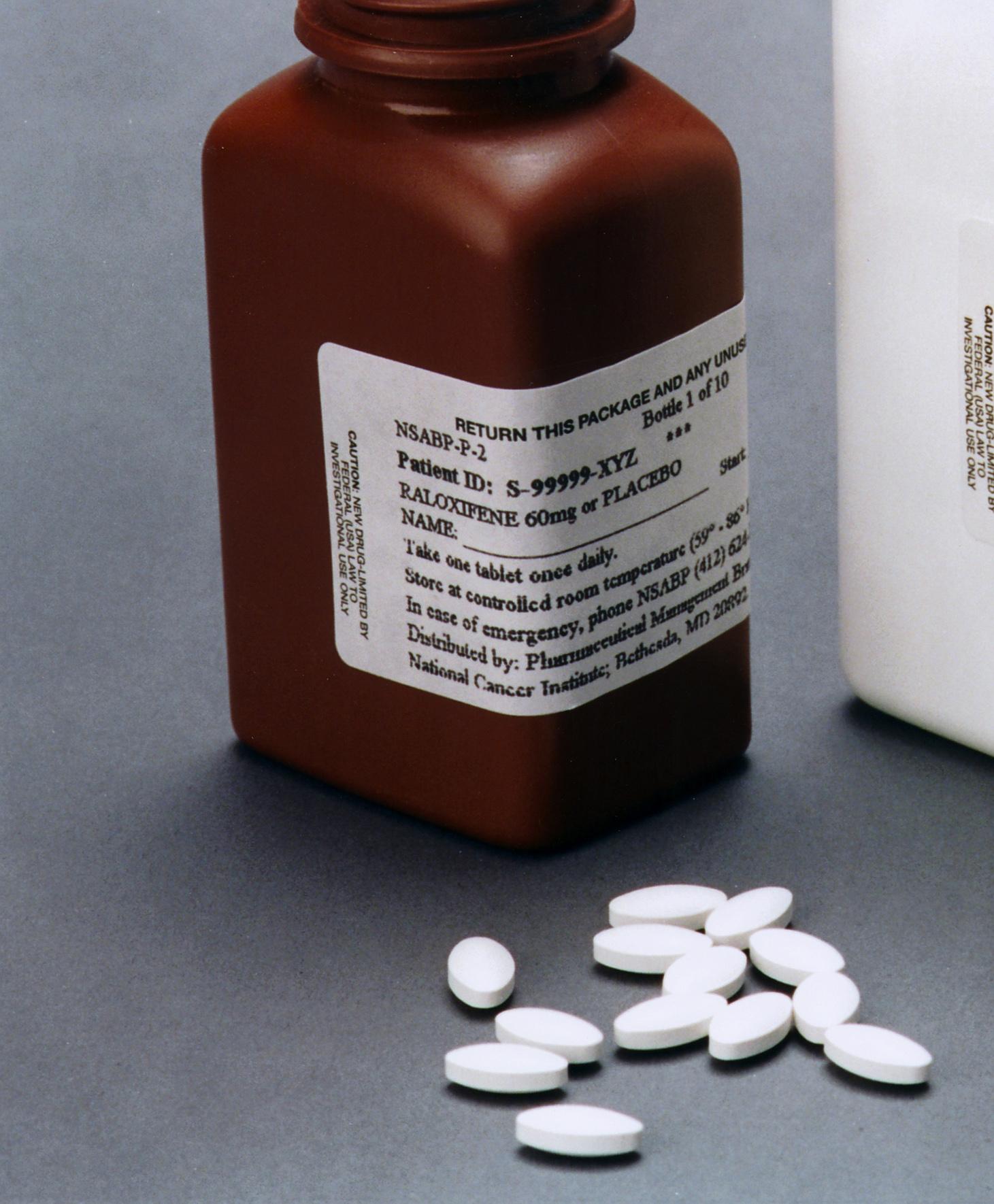
Raloxifene
Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth. Common side effects include hot flashes, leg cramps, swelling, and joint pain. Severe side effects may include blood clots and stroke. Use during pregnancy may harm the baby. The medication may worsen menstrual symptoms. Raloxifene is a selective estrogen receptor modulator (SERM) and therefore a mixed agonist– antagonist of the estrogen receptor (ER). It has estrogenic effects in bone and antiestrogenic effects in the breasts and uterus. Raloxifene was approved for medical use in the United States in 1997. It is available as a generic medication. In 2020, it was the 292nd most commonly prescribed medication in the United States, with more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
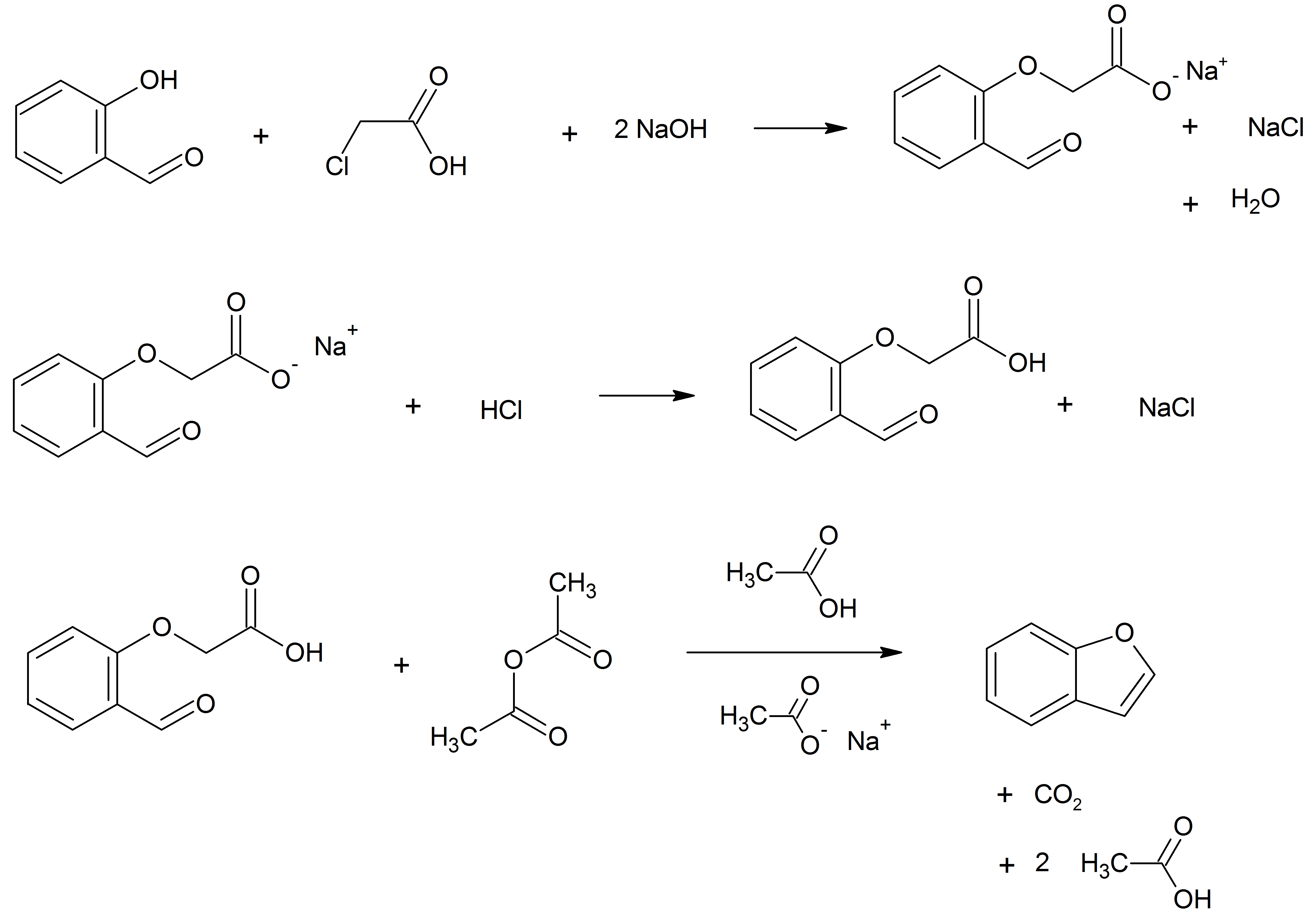
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BTCP
Benocyclidine, also known as benzothiophenylcyclohexylpiperidine (BTCP), is a psychoactive recreational drug of the arylcyclohexylamine class which is related to phencyclidine (PCP). It was first described in a patent application naming Marc Caron and colleagues at Duke University in 1997. It acts as a potent and selective dopamine reuptake inhibitor (DRI) and a psychostimulant. Unlike related compounds like phencyclidine and ketamine, benocyclidine is a pure DRI with negligible affinity for the NMDA receptor, and it therefore lacks any anticonvulsant, anesthetic, hallucinogenic, or dissociative effects. It has been used to label the dopamine transporter. BCP was used to try to find a common pharmacophore for DRI type stimulants. More recently, benocyclidine has been found in several ecstasy tablets, sold as MDMA. Legal status in the United States Benocyclidine is not scheduled at the federal level in the United States, but may be considered an analog of PCP, in which case pur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indene
Indene is a flammable polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This aromatic liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac. Isolation Indene occurs naturally in coal-tar fractions boiling around 175–185 °C. It can be obtained by heating this fraction with sodium to precipitate solid "sodio-indene". This step exploits indene's weak acidity evidenced by its deprotonation by sodium to give the indenyl derivative. The sodio-indene is converted back to indene by steam distillation. Reactivity Indene readily polymerises. Oxidation of indene with acid dichromate yields homophthalic acid (''o''-carboxylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzo(c)thiophene
Benzo 'c''hiophene is an organic compound with the chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ... C8H6S. The similarly named Benzo hiophene is an isomer with the sulfur in the position adjacent to the benzene ring. Benzo hiophene is more stable and far more commonly encountered. References Benzo(c)thiophenes Simple aromatic rings {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zileuton
Zileuton (trade name Zyflo) is an orally active inhibitor of 5-lipoxygenase, and thus inhibits leukotrienes (LTB4, LTC4, LTD4, and LTE4) formation, used for the maintenance treatment of asthma. Zileuton was introduced in 1996 by Abbott Laboratories and is now marketed in two formulations by Cornerstone Therapeutics Inc. under the brand names Zyflo and Zyflo CR. The original immediate-release formulation, Zyflo, is taken four times per day. The extended-release formulation, Zyflo CR, is taken twice daily. Although the 600 mg immediate release tablet (Zyflo) and extended release formulation of zileuton are still available (Zyflo CR), the 300 mg immediate release tablet was withdrawn from the U.S. market on February 12, 2008. Pharmacotherapy Indications and dosing Zileuton is indicated for the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older. Zileuton is not indicated for use in the reversal of bronchospasm in acute asthma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the '' thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. " Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |