Chemical Changes on:
[Wikipedia]
[Google]
[Amazon]
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the

 Retrosynthetic analysis can be applied to design a complex synthesis reaction. Here the analysis starts from the products, for example by splitting selected chemical bonds, to arrive at plausible initial reagents. A special arrow (⇒) is used in retro reactions.
Retrosynthetic analysis can be applied to design a complex synthesis reaction. Here the analysis starts from the products, for example by splitting selected chemical bonds, to arrive at plausible initial reagents. A special arrow (⇒) is used in retro reactions.
 The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a dissociation into one or more other molecules. Such reactions require the addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–trans isomerization, in which the cis-form of a compound converts to the trans-form or vice versa.
In a typical dissociation reaction, a bond in a molecule splits (ruptures) resulting in two molecular fragments. The splitting can be homolytic or heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ions. Dissociation plays an important role in triggering chain reactions, such as hydrogen–oxygen or
The most important elementary reactions are unimolecular and bimolecular reactions. Only one molecule is involved in a unimolecular reaction; it is transformed by isomerization or a dissociation into one or more other molecules. Such reactions require the addition of energy in the form of heat or light. A typical example of a unimolecular reaction is the cis–trans isomerization, in which the cis-form of a compound converts to the trans-form or vice versa.
In a typical dissociation reaction, a bond in a molecule splits (ruptures) resulting in two molecular fragments. The splitting can be homolytic or heterolytic. In the first case, the bond is divided so that each product retains an electron and becomes a neutral radical. In the second case, both electrons of the chemical bond remain with one of the products, resulting in charged ions. Dissociation plays an important role in triggering chain reactions, such as hydrogen–oxygen or AB -> A + B
: Dissociation of a molecule AB into fragments A and B
For A + B -> AB
Another possibility is that only a portion of one molecule is transferred to the other molecule. This type of reaction occurs, for example, in HA + B -> A + HB
for example
:NaCl + AgNO3 -> NaNO3 + AgCl(v)
2CO(g) + MoO2(s) -> 2CO2(g) + Mo(s) ;
This reaction to form carbon dioxide and molybdenum is endothermic at low temperatures, becoming less so with increasing temperature. Δ''H''° is zero at , and the reaction becomes exothermic above that temperature.
Changes in temperature can also reverse the direction tendency of a reaction. For example, the water gas shift reaction
:CO(g) + H2O() <=> CO2(g) + H2(g)
is favored by low temperatures, but its reverse is favored by high temperatures. The shift in reaction direction tendency occurs at .
Reactions can also be characterized by their internal energy change, which takes into account changes in the entropy, volume and chemical potentials. The latter depends, among other things, on the activity (chemistry), activities of the involved substances.
:;
:: : internal energy, : entropy, : pressure, : chemical potential, : number of molecules, : differential calculus, small change sign

A + B->AB
Two or more reactants yielding one product is another way to identify a synthesis reaction. One example of a synthesis reaction is the combination of iron and 8Fe + S8->8FeS
Another example is simple hydrogen gas combined with simple oxygen gas to produce a more complex substance, such as water.To react or not to react?
Utah State Office of Education. Retrieved 4 June 2011.
AB->A + B
One example of a decomposition reaction is the electrolysis of water to make oxygen and hydrogen gas:
2H2O->2H2 + O2
A + BC->AC + B
One example of a single displacement reaction is when magnesium replaces hydrogen in water to make solid magnesium hydroxide and hydrogen gas:
Mg + 2H2O->Mg(OH)2 (v) + H2 (^)
AB + CD->AD + CB
For example, when barium chloride (BaCl2) and magnesium sulfate (MgSO4) react, the SO42− anion switches places with the 2Cl− anion, giving the compounds BaSO4 and MgCl2.
Another example of a double displacement reaction is the reaction of lead(II) nitrate with potassium iodide to form lead(II) iodide and potassium nitrate:
Pb(NO3)2 + 2KI->PbI2(v) + 2KNO3
C8H18(l) + 25/2 O2(g)->8CO2 + 9H2O(l)
releases 5500 kJ. A combustion reaction can also result from carbon, magnesium or 2Mg(s) + O2->2MgO(s)
S(s) + O2(g)->SO2(g)
2Na(s) + Cl2(g)->2NaCl(s)
In the reaction, sodium metal goes from an oxidation state of 0 (a pure element) to +1: in other words, the sodium lost one electron and is said to have been oxidized. On the other hand, the chlorine gas goes from an oxidation of 0 (also a pure element) to −1: the chlorine gains one electron and is said to have been reduced. Because the chlorine is the one reduced, it is considered the electron acceptor, or in other words, induces oxidation in the sodium – thus the chlorine gas is considered the oxidizing agent. Conversely, the sodium is oxidized or is the electron donor, and thus induces a reduction in the other species and is considered the ''reducing agent''.
Which of the involved reactants would be a reducing or oxidizing agent can be predicted from the electronegativity of their elements. Elements with low electronegativities, such as most metals, easily donate electrons and oxidize – they are reducing agents. On the contrary, many oxides or ions with high oxidation numbers of their non-oxygen atoms, such as , , , , or , can gain one or two extra electrons and are strong oxidizing agents.
For some main-group elements the number of electrons donated or accepted in a redox reaction can be predicted from the electron configuration of the reactant element. Elements try to reach the low-energy noble gas configuration, and therefore Alkali metal, alkali metals and Halogen, halogens will donate and accept one electron, respectively. Noble gases themselves are chemically inactive.
The overall redox reaction Electrochemistry#Balancing redox reactions, can be balanced by combining the oxidation and reduction half-reactions multiplied by coefficients such that the number of electrons lost in the oxidation equals the number of electrons gained in the reduction.
An important class of redox reactions are the electrolytic Electrochemistry, electrochemical reactions, where electrons from the power supply at the negative electrode are used as the reducing agent and electron withdrawal at the positive electrode as the oxidizing agent. These reactions are particularly important for the production of chemical elements, such as chlorine or aluminium. The reverse process, in which electrons are released in redox reactions and chemical energy is converted to electrical energy, is possible and used in Electric battery, batteries.
 In complexation reactions, several ligands react with a metal atom to form a coordination complex. This is achieved by providing lone pairs of the ligand into empty Atomic orbital, orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18
In complexation reactions, several ligands react with a metal atom to form a coordination complex. This is achieved by providing lone pairs of the ligand into empty Atomic orbital, orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18
\underset + \underset <=> \underset + \underset
The reverse reaction is possible, and thus the acid/base and conjugated base/acid are always in equilibrium. The equilibrium is determined by the acid dissociation constant, acid and base dissociation constants (''K''a and ''K''b) of the involved substances. A special case of the acid-base reaction is the neutralization (chemistry), neutralization where an acid and a base, taken at the exact same amounts, form a neutral Salt (chemistry), salt.
Acid-base reactions can have different definitions depending on the acid-base concept employed. Some of the most common are:
* Acid–base reaction#Arrhenius definition, Arrhenius definition: Acids dissociate in water releasing H3O+ ions; bases dissociate in water releasing OH− ions.
* Brønsted–Lowry acid–base theory, Brønsted–Lowry definition: Acids are proton (H+) donors, bases are proton acceptors; this includes the Arrhenius definition.
* Acid–base reaction#Lewis definition, Lewis definition: Acids are electron-pair acceptors, and bases are electron-pair donors; this includes the Brønsted-Lowry definition.
 Precipitation (chemistry), Precipitation is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the solubility limit and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by the removal of the solvent. Rapid precipitation results in an amorphous or microcrystalline residue and a slow process can yield single crystals. The latter can also be obtained by Recrystallization (chemistry), recrystallization from microcrystalline salts.
Precipitation (chemistry), Precipitation is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the solubility limit and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by the removal of the solvent. Rapid precipitation results in an amorphous or microcrystalline residue and a slow process can yield single crystals. The latter can also be obtained by Recrystallization (chemistry), recrystallization from microcrystalline salts.
 In Photochemistry, photochemical reactions, atoms and molecules absorb energy (photons) of the illumination light and convert it into an excited state. They can then release this energy by breaking chemical bonds, thereby producing radicals. Photochemical reactions include hydrogen–oxygen reactions, radical polymerization, chain reactions and rearrangement reactions.
Many important processes involve photochemistry. The premier example is photosynthesis, in which most plants use solar energy to convert carbon dioxide and water into glucose, disposing of oxygen as a side-product. Humans rely on photochemistry for the formation of vitamin D, and visual perception, vision is initiated by a photochemical reaction of rhodopsin. In fireflies, an
In Photochemistry, photochemical reactions, atoms and molecules absorb energy (photons) of the illumination light and convert it into an excited state. They can then release this energy by breaking chemical bonds, thereby producing radicals. Photochemical reactions include hydrogen–oxygen reactions, radical polymerization, chain reactions and rearrangement reactions.
Many important processes involve photochemistry. The premier example is photosynthesis, in which most plants use solar energy to convert carbon dioxide and water into glucose, disposing of oxygen as a side-product. Humans rely on photochemistry for the formation of vitamin D, and visual perception, vision is initiated by a photochemical reaction of rhodopsin. In fireflies, an
 In catalysis, the reaction does not proceed directly, but through a reaction with a third substance known as catalyst. Although the catalyst takes part in the reaction, forming weak bonds with reactants or intermediates, it is returned to its original state by the end of the reaction and so is not consumed. However, it can be inhibited, deactivated or destroyed by secondary processes. Catalysts can be used in a different phase (heterogeneous catalysis, heterogeneous) or in the same phase (homogeneous catalysis, homogeneous) as the reactants. In heterogeneous catalysis, typical secondary processes include coking where the catalyst becomes covered by polymeric side products. Additionally, heterogeneous catalysts can dissolve into the solution in a solid-liquid system or evaporate in a solid–gas system. Catalysts can only speed up the reaction – chemicals that slow down the reaction are called inhibitors. Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons. With a catalyst, a reaction that is kinetically inhibited by high activation energy can take place in the circumvention of this activation energy.
Heterogeneous catalysts are usually solids, powdered in order to maximize their surface area. Of particular importance in heterogeneous catalysis are the platinum group metals and other transition metals, which are used in hydrogenations, catalytic reforming and in the synthesis of commodity chemicals such as
In catalysis, the reaction does not proceed directly, but through a reaction with a third substance known as catalyst. Although the catalyst takes part in the reaction, forming weak bonds with reactants or intermediates, it is returned to its original state by the end of the reaction and so is not consumed. However, it can be inhibited, deactivated or destroyed by secondary processes. Catalysts can be used in a different phase (heterogeneous catalysis, heterogeneous) or in the same phase (homogeneous catalysis, homogeneous) as the reactants. In heterogeneous catalysis, typical secondary processes include coking where the catalyst becomes covered by polymeric side products. Additionally, heterogeneous catalysts can dissolve into the solution in a solid-liquid system or evaporate in a solid–gas system. Catalysts can only speed up the reaction – chemicals that slow down the reaction are called inhibitors. Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons. With a catalyst, a reaction that is kinetically inhibited by high activation energy can take place in the circumvention of this activation energy.
Heterogeneous catalysts are usually solids, powdered in order to maximize their surface area. Of particular importance in heterogeneous catalysis are the platinum group metals and other transition metals, which are used in hydrogenations, catalytic reforming and in the synthesis of commodity chemicals such as
 In the third type of substitution reaction, radical substitution, the attacking particle is a radical. This process usually takes the form of a chain reaction, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.
:;
In the third type of substitution reaction, radical substitution, the attacking particle is a radical. This process usually takes the form of a chain reaction, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.
:;X. + R-H -> X-H + R.
:;R. + X2 -> R-X + X.
:: Reactions during the chain reaction of radical substitution
 The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
 The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the electrophilic addition of hydrogen bromide, an electrophile (proton) attacks the double bond forming a carbocation, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule. This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the hydroboration–oxidation reaction, wherein the first step, the boron atom acts as electrophile and adds to the less substituted carbon atom. In the second step, the nucleophilic hydroperoxide or halogen anion attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the nucleophilic addition plays an important role in the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with elimination so that after the reaction the carbonyl group is present again. It is, therefore, called an addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
The counterpart of elimination is an addition where double or triple bonds are converted into single bonds. Similar to substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the electrophilic addition of hydrogen bromide, an electrophile (proton) attacks the double bond forming a carbocation, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule. This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the hydroboration–oxidation reaction, wherein the first step, the boron atom acts as electrophile and adds to the less substituted carbon atom. In the second step, the nucleophilic hydroperoxide or halogen anion attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the nucleophilic addition plays an important role in the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with elimination so that after the reaction the carbonyl group is present again. It is, therefore, called an addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
 Nucleophilic addition of a carbanion or another nucleophile to the double bond of an Α,β-unsaturated carbonyl compound, alpha, beta-unsaturated carbonyl compound can proceed via the Michael reaction, which belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds.
Some additions which can not be executed with nucleophiles and electrophiles can be succeeded with free radicals. As with the free-radical substitution, the radical addition proceeds as a chain reaction, and such reactions are the basis of the Radical polymerization, free-radical polymerization.
Nucleophilic addition of a carbanion or another nucleophile to the double bond of an Α,β-unsaturated carbonyl compound, alpha, beta-unsaturated carbonyl compound can proceed via the Michael reaction, which belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds.
Some additions which can not be executed with nucleophiles and electrophiles can be succeeded with free radicals. As with the free-radical substitution, the radical addition proceeds as a chain reaction, and such reactions are the basis of the Radical polymerization, free-radical polymerization.
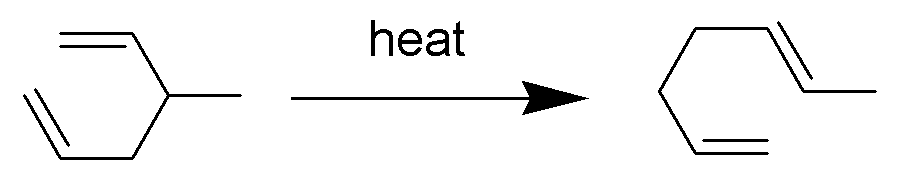 In a rearrangement reaction, the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. These include Sigmatropic reaction, hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloadditions and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction (the so-called [4+2] cycloaddition) between a conjugated diene and a substituted alkene to form a substituted cyclohexene system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereochemistry, stereoisomeric products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules.
In a rearrangement reaction, the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. These include Sigmatropic reaction, hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloadditions and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction (the so-called [4+2] cycloaddition) between a conjugated diene and a substituted alkene to form a substituted cyclohexene system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereochemistry, stereoisomeric products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules.
 Biochemistry, Biochemical reactions are mainly controlled by complex proteins called enzymes, which are usually specialized to Catalysis, catalyze only a single, specific reaction. The reaction takes place in the active site, a small part of the enzyme which is usually found in a cleft or pocket lined by amino acid residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relative to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as metabolism. Among the most important of its mechanisms is the anabolism, in which different DNA and enzyme-controlled processes result in the production of large molecules such as proteins and carbohydrates from smaller units. Bioenergetics studies the sources of energy for such reactions. Important energy sources are glucose and Dioxygen in biological reactions, oxygen, which can be produced by plants via photosynthesis or assimilated from food and air, respectively. All organisms use this energy to produce adenosine triphosphate (ATP), which can then be used to energize other reactions. Decomposition of organic material by Fungus, fungi, bacteria and other Microorganism, micro-organisms is also within the scope of
Biochemistry, Biochemical reactions are mainly controlled by complex proteins called enzymes, which are usually specialized to Catalysis, catalyze only a single, specific reaction. The reaction takes place in the active site, a small part of the enzyme which is usually found in a cleft or pocket lined by amino acid residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relative to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as metabolism. Among the most important of its mechanisms is the anabolism, in which different DNA and enzyme-controlled processes result in the production of large molecules such as proteins and carbohydrates from smaller units. Bioenergetics studies the sources of energy for such reactions. Important energy sources are glucose and Dioxygen in biological reactions, oxygen, which can be produced by plants via photosynthesis or assimilated from food and air, respectively. All organisms use this energy to produce adenosine triphosphate (ATP), which can then be used to energize other reactions. Decomposition of organic material by Fungus, fungi, bacteria and other Microorganism, micro-organisms is also within the scope of
 Chemical reactions are central to chemical engineering, where they are used for the synthesis of new compounds from natural raw materials such as petroleum, mineral ores, and oxygen in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalysts are especially helpful for reducing the energy required for the reaction and increasing its reaction rate.
Some specific reactions have their niche applications. For example, the thermite reaction is used to generate light and heat in pyrotechnics and welding. Although it is less controllable than the more conventional Oxy-fuel welding and cutting, oxy-fuel welding, arc welding and flash welding, it requires much less equipment and is still used to mend rails, especially in remote areas.
Chemical reactions are central to chemical engineering, where they are used for the synthesis of new compounds from natural raw materials such as petroleum, mineral ores, and oxygen in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalysts are especially helpful for reducing the energy required for the reaction and increasing its reaction rate.
Some specific reactions have their niche applications. For example, the thermite reaction is used to generate light and heat in pyrotechnics and welding. Although it is less controllable than the more conventional Oxy-fuel welding and cutting, oxy-fuel welding, arc welding and flash welding, it requires much less equipment and is still used to mend rails, especially in remote areas.
 A chemical reaction is a process that leads to the
A chemical reaction is a process that leads to the chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
transformation of one set of chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
s to another. When chemical reactions occur, the atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
reactions encompass changes that only involve the positions of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in the forming and breaking of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s between atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable
In dynamical systems instability means that some of the outputs or internal state (controls), states increase with time, without bounds. Not all systems that are not Stability theory, stable are unstable; systems can also be marginal stability ...
and radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
elements where both electronic and nuclear changes can occur.
The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form Product (chemistry), products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be element ...
s, and the information on the precise course of action is part of the reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
. Chemical reactions are described with chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.
Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Some reactions produce heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
and are called exothermic reactions, while others may require heat to enable the reaction to occur, which are called endothermic reactions. Typically, reaction rates increase with increasing temperature because there is more thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
available to reach the activation energy necessary for breaking bonds between atoms.
A reaction may be classified as redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
in which oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
and reduction occur or non-redox in which there is no oxidation and reduction occurring. Most simple redox reactions may be classified as a combination, decomposition, or single displacement reaction.
Different chemical reactions are used during chemical synthesis in order to obtain the desired product. In biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are ...
s. These reactions are often catalyzed by protein enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s. Enzymes increase the rates of biochemical reactions, so that metabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
syntheses and decompositions impossible under ordinary conditions can occur at the temperature and concentrations present within a cell.
The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
s and reactions between elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a c ...
s, as described by quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
.
History
Chemical reactions such as combustion in fire,fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
and the reduction of ores to metals were known since antiquity. Initial theories of transformation of materials were developed by Greek philosophers, such as the Four-Element Theory of Empedocles
Empedocles (; ; , 444–443 BC) was a Ancient Greece, Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is known best for originating the Cosmogony, cosmogonic theory of the four cla ...
stating that any substance is composed of the four basic elements – fire, water, air and earth. In the Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the 5th to the late 15th centuries, similarly to the post-classical period of global history. It began with the fall of the Western Roman Empire and ...
, chemical transformations were studied by alchemists. They attempted, in particular, to convert lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
into gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
, for which purpose they used reactions of lead and lead-copper alloys with sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
.
The artificial production of chemical substances already was a central goal for medieval alchemists. Examples include the synthesis of ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
from organic substances as described in the works (c. 850–950) attributed to Jābir ibn Ḥayyān, or the production of mineral acids such as sulfuric and nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
s by later alchemists, starting from c. 1300. The production of mineral acids involved the heating of sulfate and nitrate minerals such as copper sulfate, alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , such that is a valence (chemistry), monovalent cation such as potassium ...
and saltpeter. In the 17th century, Johann Rudolph Glauber produced hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 mill ...
by reacting sulfuric acid and sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
. With the development of the lead chamber process in 1746 and the Leblanc process, allowing large-scale production of sulfuric acid and sodium carbonate, respectively, chemical reactions became implemented into the industry. Further optimization of sulfuric acid technology resulted in the contact process in the 1880s, and the Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
was developed in 1909–1910 for ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
synthesis.
From the 16th century, researchers including Jan Baptist van Helmont
Jan Baptist van Helmont ( , ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to be ...
, Robert Boyle, and Isaac Newton
Sir Isaac Newton () was an English polymath active as a mathematician, physicist, astronomer, alchemist, theologian, and author. Newton was a key figure in the Scientific Revolution and the Age of Enlightenment, Enlightenment that followed ...
tried to establish theories of experimentally observed chemical transformations. The phlogiston theory was proposed in 1667 by Johann Joachim Becher. It postulated the existence of a fire-like element called "phlogiston", which was contained within combustible bodies and released during combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
. This proved to be false in 1785 by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
who found the correct explanation of the combustion as a reaction with oxygen from the air.
Joseph Louis Gay-Lussac recognized in 1808 that gases always react in a certain relationship with each other. Based on this idea and the atomic theory of John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched Color blindness, colour blindness; as a result, the umbrella term ...
, Joseph Proust
Joseph Louis Proust (26 September 1754 – 5 July 1826) was a French people, French chemist. He was best known for his discovery of the law of definite proportions in 1797, stating that chemical compounds always combine in constant proportions.
...
had developed the law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's law or the law of constant composition, states that a given
chemical compound contains its constituent elements in a fixed ratio (by mass) and does not depend on its source ...
, which later resulted in the concepts of stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must ...
and chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s.
Regarding the organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, it was long believed that compounds obtained from living organisms were too complex to be obtained synthetically. According to the concept of vitalism
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
, organic matter was endowed with a "vital force" and distinguished from inorganic materials. This separation was ended however by the synthesis of urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
from inorganic precursors by Friedrich Wöhler in 1828. Other chemists who brought major contributions to organic chemistry include Alexander William Williamson with his synthesis of ethers and Christopher Kelk Ingold, who, among many discoveries, established the mechanisms of substitution reactions.
Characteristics
The general characteristics of chemical reactions are: * Evolution of a gas * Formation of a precipitate * Change intemperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
* Change in state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
Equations

Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
s are used to graphically illustrate chemical reactions. They consist of chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
or structural formulas of the reactants on the left and those of the products on the right. They are separated by an arrow (→) which indicates the direction and type of the reaction; the arrow is read as the word "yields". The tip of the arrow points in the direction in which the reaction proceeds. A double arrow () pointing in opposite directions is used for equilibrium reactions. Equations should be balanced according to the stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must ...
, the number of atoms of each species should be the same on both sides of the equation. This is achieved by scaling the number of involved molecules (A, B, C and D in a schematic example below) by the appropriate integers ''a, b, c'' and ''d''.
:
More elaborate reactions are represented by reaction schemes, which in addition to starting materials and products show important intermediates or transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s. Also, some relatively minor additions to the reaction can be indicated above the reaction arrow; examples of such additions are water, heat, illumination, a catalyst, etc. Similarly, some minor products can be placed below the arrow, often with a minus sign.
Elementary reactions
Theelementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form Product (chemistry), products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be element ...
is the smallest division into which a chemical reaction can be decomposed, it has no intermediate products. Most experimentally observed reactions are built up from many elementary reactions that occur in parallel or sequentially. The actual sequence of the individual elementary reactions is known as reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
. An elementary reaction involves a few molecules, usually one or two, because of the low probability for several molecules to meet at a certain time.
polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
reactions.
:bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary reaction, elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of Sto ...
reactions, two molecules collide and react with each other. Their merger is called chemical synthesis or an addition reaction.
:redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
and acid-base reactions. In redox reactions, the transferred particle is an electron, whereas in acid-base reactions it is a proton. This type of reaction is also called metathesis.
:Chemical equilibrium
Most chemical reactions are reversible; that is, they can and do run in both directions. The forward and reverse reactions are competing with each other and differ in reaction rates. These rates depend on the concentration and therefore change with the time of the reaction: the reverse rate gradually increases and becomes equal to the rate of the forward reaction, establishing the so-called chemical equilibrium. The time to reach equilibrium depends on parameters such as temperature, pressure, and the materials involved, and is determined by the minimum free energy. In equilibrium, theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of reaction must be zero. The pressure dependence can be explained with the Le Chatelier's principle. For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with fewer moles of gas.
The reaction yield stabilizes at equilibrium but can be increased by removing the product from the reaction mixture or changed by increasing the temperature or pressure. A change in the concentrations of the reactants does not affect the equilibrium constant but does affect the equilibrium position.
Thermodynamics
Chemical reactions are determined by the laws ofthermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
. Reactions can proceed by themselves if they are exergonic, that is if they release free energy. The associated free energy change of the reaction is composed of the changes of two different thermodynamic quantities, enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
and entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
:
:; .
:: : free energy, : enthalpy, : temperature, : entropy, : difference (change between original and product)
Reactions can be exothermic, where Δ''H'' is negative and energy is released. Typical examples of exothermic reactions are combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
, precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
and crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, in which ordered solids are formed from disordered gaseous or liquid phases. In contrast, in endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
reactions, heat is consumed from the environment. This can occur by increasing the entropy of the system, often through the formation of gaseous or dissolved reaction products, which have higher entropy. Since the entropy term in the free-energy change increases with temperature, many endothermic reactions preferably take place at high temperatures. On the contrary, many exothermic reactions such as crystallization occur preferably at lower temperatures. A change in temperature can sometimes reverse the sign of the enthalpy of a reaction, as for the carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
reduction of molybdenum dioxide:
:Kinetics
The speed at which reactions take place is studied by Chemical kinetics, reaction kinetics. The rate depends on various parameters, such as: * Reactant concentrations, which usually make the reaction happen at a faster rate if raised through increased collisions per unit of time. Some reactions, however, have rates that are ''independent'' of reactant concentrations, due to a limited number of catalytic sites. These are called Rate law#Zero-order reactions, zero order reactions. * Surface area available for contact between the reactants, in particular solid ones in heterogeneous systems. Larger surface areas lead to higher reaction rates. * Pressure – increasing the pressure decreases the volume between molecules and therefore increases the frequency of collisions between the molecules. * Activation energy, which is defined as the amount of energy required to make the reaction start and carry on spontaneously. Higher activation energy implies that the reactants need more energy to start than a reaction with lower activation energy. * Temperature, which hastens reactions if raised, since higher temperature increases the energy of the molecules, creating more collisions per unit of time, * The presence or absence of a catalyst. Catalysts are substances that make weak bonds with reactants or intermediates and change the pathway (mechanism) of a reaction which in turn increases the speed of a reaction by lowering the activation energy needed for the reaction to take place. A catalyst is not destroyed or changed during a reaction, so it can be used again. * For some reactions, the presence of electromagnetic radiation, most notably ultraviolet light, is needed to promote the breaking of bonds to start the reaction. This is particularly true for reactions involving radical (chemistry), radicals. Several theories allow calculating the reaction rates at the molecular level. This field is referred to as reaction dynamics. The rate ''v'' of a Rate equation#First-order reactions, first-order reaction, which could be the disintegration of a substance A, is given by: : Its integration yields: : Here ''k'' is the first-order rate constant, having dimension 1/time, [A](''t'') is the concentration at a time ''t'' and [A]0 is the initial concentration. The rate of a first-order reaction depends only on the concentration and the properties of the involved substance, and the reaction itself can be described with a characteristic half-life. More than one time constant is needed when describing reactions of higher order. The temperature dependence of the rate constant usually follows the Arrhenius equation: : where ''E''a is the activation energy and ''k''B is the Boltzmann constant. One of the simplest models of reaction rate is the collision theory. More realistic models are tailored to a specific problem and include the transition state theory, the calculation of the potential energy surface, the Marcus theory and the RRKM theory, Rice–Ramsperger–Kassel–Marcus (RRKM) theory.Reaction types
Four basic types
Synthesis
In a synthesis reaction, two or more simple substances combine to form a more complex substance. These reactions are in the general form:sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
to form iron(II) sulfide:
Utah State Office of Education. Retrieved 4 June 2011.
Decomposition
A decomposition reaction is when a more complex substance breaks down into its more simple parts. It is thus the opposite of a synthesis reaction and can be written asSingle displacement
In a single displacement reaction, a single uncombined element replaces another in a compound; in other words, one element trades places with another element in a compound. These reactions come in the general form of:Double displacement
In a double replacement reaction, double displacement reaction, the anions and cations of two compounds switch places and form two entirely different compounds. These reactions are in the general form:Forward and backward reactions
According to Le Chatelier's Principle, reactions may proceed in the forward or reverse direction until they end or reach Equilibrium chemistry, equilibrium.Forward reactions
Reactions that proceed in the forward direction (from left to right) to approach equilibrium are often called spontaneous reactions, that is, is negative, which means that if they occur at constant temperature and pressure, they decrease theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of the reaction. They require less energy to proceed in the forward direction. Reactions are usually written as forward reactions in the direction in which they are spontaneous. Examples:
* Reaction of hydrogen and oxygen to form water.
: +
* Dissociation of acetic acid in water into acetate ions and hydronium ions.
: + +
Backward reactions
Reactions that proceed in the backward direction to approach equilibrium are often called non-spontaneous reactions, that is, is positive, which means that if they occur at constant temperature and pressure, they increase theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of the reaction. They require input of energy to proceed in the forward direction. Examples include:
* Charging a normal DC battery (consisting of electrolytic cells) from an external electrical power source
* Photosynthesis driven by absorption of electromagnetic radiation usually in the form of sunlight
: + + → +
Combustion
In acombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
reaction, an element or compound reacts with an oxidant, usually oxygen, often producing energy in the form of heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
or light. Combustion reactions frequently involve a hydrocarbon. For instance, the combustion of 1 mole (114 g) of octane in oxygen
sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
reacting with oxygen.
Oxidation and reduction
Redox reactions can be understood in terms of the transfer of electrons from one involved species (reducing agent) to another (oxidizing agent). In this process, the former species is ''oxidized'' and the latter is ''reduced''. Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation is better defined as an increase in oxidation state of atoms and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always change the oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent bonds). In the following redox reaction, hazardous sodium metal reacts with toxic chlorine gas to form the ionic compoundsodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
, or common table salt:
Complexation
 In complexation reactions, several ligands react with a metal atom to form a coordination complex. This is achieved by providing lone pairs of the ligand into empty Atomic orbital, orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18
In complexation reactions, several ligands react with a metal atom to form a coordination complex. This is achieved by providing lone pairs of the ligand into empty Atomic orbital, orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18 electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, whereas the symmetry of the resulting complex can be predicted with the crystal field theory and ligand field theory. Complexation reactions also include ligand exchange, in which one or more ligands are replaced by another, and redox processes which change the oxidation state of the central metal atom.
Acid–base reactions
In the Brønsted–Lowry acid–base theory, an acid–base reaction involves a transfer of protons (H+) from one species (the acid) to another (the base (chemistry), base). When a proton is removed from an acid, the resulting species is termed that acid's conjugate acid, conjugate base. When the proton is accepted by a base, the resulting species is termed that base's conjugate acid. In other words, acids act as proton donors and bases act as proton acceptors according to the following equation:Precipitation
Solid-state reactions
Reactions can take place between two solids. However, because of the relatively small diffusion rates in solids, the corresponding chemical reactions are very slow in comparison to liquid and gas phase reactions. They are accelerated by increasing the reaction temperature and finely dividing the reactant to increase the contacting surface area.Reactions at the solid/gas interface
The reaction can take place at the solid, gas interface, surfaces at very low pressure such as ultra-high vacuum. Via scanning tunneling microscopy, it is possible to observe reactions at the solid, gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid, gas interface are in some cases related to catalysis.Photochemical reactions
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the abdomen catalyzes a reaction that results in bioluminescence. Many significant photochemical reactions, such as ozone formation, occur in the Earth atmosphere and constitute atmospheric chemistry.
Catalysis
nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. Acids are an example of a homogeneous catalyst, they increase the nucleophilicity of carbonyls, allowing a reaction that would not otherwise proceed with electrophiles. The advantage of homogeneous catalysts is the ease of mixing them with the reactants, but they may also be difficult to separate from the products. Therefore, heterogeneous catalysts are preferred in many industrial processes.
Reactions in organic chemistry
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, in addition to oxidation, reduction or acid-base reactions, a number of other reactions can take place which involves covalent bonds between carbon atoms or carbon and heteroatoms (such as oxygen, nitrogen, halogens, etc.). Many specific reactions in organic chemistry are name reactions designated after their discoverers.
One of the most industrially important reactions is the Cracking (chemistry), cracking of heavy hydrocarbons at Oil refinery, oil refineries to create smaller, simpler molecules. This process is used to manufacture gasoline. Specific types of organic reactions may be grouped by their reaction mechanisms (particularly substitution, addition and elimination) or by the types of products they produce (for example, methylation, Polymerization, polymerisation and halogenation).
Substitution
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. These reactions can be distinguished by the type of substituting species into a nucleophilic substitution, nucleophilic, electrophilic substitution, electrophilic or radical substitution. In the first type, a nucleophile, an atom or molecule with an excess of electrons and thus a negative charge or partial charge, replaces another atom or part of the "Substrate (chemistry), substrate" molecule. The electron pair from the nucleophile attacks the substrate forming a new bond, while the leaving group departs with an electron pair. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. Examples of nucleophiles are hydroxide ion, alkoxides, amines and halides. This type of reaction is found mainly in aliphatic hydrocarbons, and rarely in aromatic hydrocarbon. The latter have high electron density and enter nucleophilic aromatic substitution only with very strong Polar effect, electron withdrawing groups. Nucleophilic substitution can take place by two different mechanisms, SN1 reaction, SN1 and SN2 reaction, SN2. In their names, S stands for substitution, N for nucleophilic, and the number represents the order (chemistry), kinetic order of the reaction, unimolecular or bimolecular. The SN1 reaction proceeds in two steps. First, the leaving group is eliminated creating a carbocation. This is followed by a rapid reaction with the nucleophile. In the SN2 mechanisms, the nucleophile forms a transition state with the attacked molecule, and only then the leaving group is cleaved. These two mechanisms differ in the stereochemistry of the products. SN1 leads to the non-stereospecific addition and does not result in a chiral center, but rather in a set of Cis–trans isomerism, geometric isomers (''cis/trans''). In contrast, a reversal (Walden inversion) of the previously existing stereochemistry is observed in the SN2 mechanism. Electrophilic substitution is the counterpart of the nucleophilic substitution in that the attacking atom or molecule, an electrophile, has low electron density and thus a positive charge. Typical electrophiles are the carbon atom of carbonyl groups, carbocations orsulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
or nitronium cations. This reaction takes place almost exclusively in aromatic hydrocarbons, where it is called electrophilic aromatic substitution. The electrophile attack results in the so-called σ-complex, a transition state in which the aromatic system is abolished. Then, the leaving group, usually a proton, is split off and the aromaticity is restored. An alternative to aromatic substitution is electrophilic aliphatic substitution. It is similar to the nucleophilic aliphatic substitution and also has two major types, SE1 and SE2.
Addition and elimination
The Addition reaction, addition and its counterpart, the elimination reaction, elimination, are reactions that change the number of substituents on the carbon atom, and form or cleave covalent bond, multiple bonds. Double bond, Double and triple bonds can be produced by eliminating a suitable leaving group. Similar to the nucleophilic substitution, there are several possible reaction mechanisms that are named after the respective reaction order. In the E1 mechanism, the leaving group is ejected first, forming a carbocation. The next step, the formation of the double bond, takes place with the elimination of a proton (deprotonation). The leaving order is reversed in the E1cb mechanism, that is the proton is split off first. This mechanism requires the participation of a base. Because of the similar conditions, both reactions in the E1 or E1cb elimination always compete with the SN1 substitution.Other organic reaction mechanisms
 In a rearrangement reaction, the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. These include Sigmatropic reaction, hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloadditions and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction (the so-called [4+2] cycloaddition) between a conjugated diene and a substituted alkene to form a substituted cyclohexene system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereochemistry, stereoisomeric products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules.
In a rearrangement reaction, the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. These include Sigmatropic reaction, hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloadditions and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reaction (the so-called [4+2] cycloaddition) between a conjugated diene and a substituted alkene to form a substituted cyclohexene system.
Whether a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in a different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereochemistry, stereoisomeric products upon cycloaddition is limited, as described by the Woodward–Hoffmann rules.
Biochemical reactions
biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
.
Applications
 Chemical reactions are central to chemical engineering, where they are used for the synthesis of new compounds from natural raw materials such as petroleum, mineral ores, and oxygen in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalysts are especially helpful for reducing the energy required for the reaction and increasing its reaction rate.
Some specific reactions have their niche applications. For example, the thermite reaction is used to generate light and heat in pyrotechnics and welding. Although it is less controllable than the more conventional Oxy-fuel welding and cutting, oxy-fuel welding, arc welding and flash welding, it requires much less equipment and is still used to mend rails, especially in remote areas.
Chemical reactions are central to chemical engineering, where they are used for the synthesis of new compounds from natural raw materials such as petroleum, mineral ores, and oxygen in air. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste. Catalysts are especially helpful for reducing the energy required for the reaction and increasing its reaction rate.
Some specific reactions have their niche applications. For example, the thermite reaction is used to generate light and heat in pyrotechnics and welding. Although it is less controllable than the more conventional Oxy-fuel welding and cutting, oxy-fuel welding, arc welding and flash welding, it requires much less equipment and is still used to mend rails, especially in remote areas.
Monitoring
Mechanisms of monitoring chemical reactions depend strongly on the reaction rate. Relatively slow processes can be analyzed in situ for the concentrations and identities of the individual ingredients. Important tools of real-time analysis are the measurement of pH and analysis of optical absorption (color) and emission spectra. A less accessible but rather efficient method is the introduction of a radioactive isotope into the reaction and monitoring how it changes over time and where it moves to; this method is often used to analyze the redistribution of substances in the human body. Faster reactions are usually studied with ultrafast laser spectroscopy where utilization of Femtochemistry, femtosecond lasers allows short-lived transition states to be monitored at a time scaled down to a few femtoseconds.#Atkins, Atkins, p. 987See also
*Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
* Chemical reaction
** Substrate (chemistry), Substrate
** Reagent
** Catalyst
** Product (chemistry), Product
* Chemical reaction model
* Chemist
* Chemistry
* Combustion
* Limiting reagent
* List of organic reactions
* Mass balance
* Microscopic reversibility
* Organic reaction
* Reaction progress kinetic analysis
* Reversible reaction
References
Bibliography
* * * * * {{DEFAULTSORT:Chemical Reaction Chemical reactions, Chemistry Change