Cation–π Interaction on:
[Wikipedia]
[Google]
[Amazon]
 Cation–π interaction is a
Cation–π interaction is a 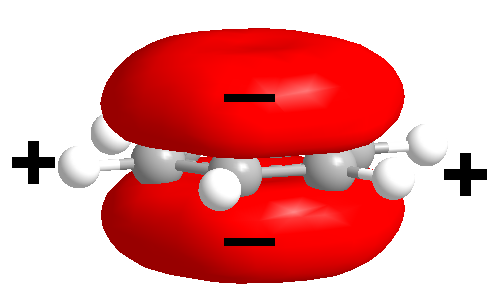
electrostatic potential maps
for a series of arenes. Electrostatic attraction is not the only component of cation–π bonding. For example, 1,3,5-trifluorobenzene interacts with cations despite having a negligible quadrupole moment. While non-electrostatic forces are present, these components remain similar over a wide variety of arenes, making the electrostatic model a useful tool in predicting relative binding energies. The other "effects" contributing to binding are not well understood.
donor-acceptor
and charge-transfer interactions have been implicated; however, energetic trends do not track well with the ability of arenes and cations to take advantage of these effects. For example, if induced dipole was a controlling effect,
K+ > Rb+ \gg Na+, Li+
 The electronic properties of the
The electronic properties of the
 An example of cation–π interactions in molecular recognition is seen in the
An example of cation–π interactions in molecular recognition is seen in the
 π-π interaction concerns the direct interactions between two &pi-systems; and cation–π interaction arises from the electrostatic interaction of a cation with the face of the π-system. Unlike these two interactions, the CH-π interaction arises mainly from charge transfer between the C-H orbital and the π-system.
A notable example of applying π-π interactions in supramolecular assembly is the synthesis of
π-π interaction concerns the direct interactions between two &pi-systems; and cation–π interaction arises from the electrostatic interaction of a cation with the face of the π-system. Unlike these two interactions, the CH-π interaction arises mainly from charge transfer between the C-H orbital and the π-system.
A notable example of applying π-π interactions in supramolecular assembly is the synthesis of 
 Cation–π interaction is a
Cation–π interaction is a noncovalent
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The ...
molecular interaction
In molecular biology, an interactome is the whole set of molecular interactions in a particular cell. The term specifically refers to physical interactions among molecules (such as those among proteins, also known as protein–protein interactions ...
between the face of an electron-rich π system (e.g. benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
, acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
) and an adjacent cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(e.g. Li+, Na+). This interaction is an example of noncovalent bonding between a monopole (cation) and a quadrupole
A quadrupole or quadrapole is one of a sequence of configurations of things like electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure re ...
(π system). Bonding energies are significant, with solution-phase values falling within the same order of magnitude as hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s and salt bridges. Similar to these other non-covalent bonds, cation–π interactions play an important role in nature, particularly in protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid ...
, molecular recognition
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
and enzyme catalysis
Enzyme catalysis is the increase in the rate of a process by an "enzyme", a biological molecule. Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, calle ...
. The effect has also been observed and put to use in synthetic systems.

Origin of the effect
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, the model π system, has no permanent dipole moment, as the contributions of the weakly polar carbon–hydrogen bonds cancel due to molecular symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
. However, the electron-rich π system above and below the benzene ring hosts a partial negative charge. A counterbalancing positive charge is associated with the plane of the benzene atoms, resulting in an electric quadrupole
A quadrupole or quadrapole is one of a sequence of configurations of things like electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure re ...
(a pair of dipoles, aligned like a parallelogram so there is no net molecular dipole moment). The negatively charged region of the quadrupole can then interact favorably with positively charged species; a particularly strong effect is observed with cations of high charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in co ...
.
Nature of the cation–π interaction
The most studied cation–π interactions involve binding between anaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
π system and an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
or nitrogenous
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
cation. The optimal interaction geometry places the cation in van der Waals contact with the aromatic ring, centered on top of the π face along the 6-fold axis. Studies have shown that electrostatics dominate interactions in simple systems, and relative binding energies correlate well with electrostatic potential energy
Electric potential energy is a potential energy (measured in joules) that results from conservative force, conservative Coulomb forces and is associated with the configuration of a particular set of point electric charge, charges within a defi ...
.
The Electrostatic Model developed by Dougherty and coworkers describes trends in binding energy based on differences in electrostatic attraction. It was found that interaction energies of cation–π pairs correlate well with electrostatic potential above the π face of arenes: for eleven Na+-aromatic adducts, the variation in binding energy between the different adducts could be completely rationalized by electrostatic differences. Practically, this allows trends to be predicted qualitatively based on visual representations oelectrostatic potential maps
for a series of arenes. Electrostatic attraction is not the only component of cation–π bonding. For example, 1,3,5-trifluorobenzene interacts with cations despite having a negligible quadrupole moment. While non-electrostatic forces are present, these components remain similar over a wide variety of arenes, making the electrostatic model a useful tool in predicting relative binding energies. The other "effects" contributing to binding are not well understood.
Polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
donor-acceptor
and charge-transfer interactions have been implicated; however, energetic trends do not track well with the ability of arenes and cations to take advantage of these effects. For example, if induced dipole was a controlling effect,
aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
compounds such as cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
should be good cation–π partners (but are not).
The cation–π interaction is noncovalent and is therefore fundamentally different than bonding between transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
and π systems. Transition metals have the ability to share electron density with π-systems through d-orbital
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calc ...
s, creating bonds that are highly covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
in character and cannot be modeled as a cation–π interaction.
Factors influencing the cation–π bond strength
Several criteria influence the strength of the bonding: the nature of the cation,solvation
Solvations describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, includi ...
effects, the nature of the π system, and the geometry of the interaction.
Nature of the cation
Fromelectrostatics
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles after triboelectric e ...
(Coulomb's law
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental scientific law, law of physics that calculates the amount of force (physics), force between two electric charge, electrically charged particles at rest. This electric for ...
), smaller and more positively charged cations lead to larger electrostatic attraction. Since cation–π interactions are predicted by electrostatics, it follows that cations with larger charge density interact more strongly with π systems.
The following table shows a series of Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of binding between benzene and several cations in the gas phase. For a singly charged species, the gas-phase interaction energy correlates with the ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cati ...
, (non-spherical ionic radii are approximate).
This trend supports the idea that coulombic forces play a central role in interaction strength, since for other types of bonding one would expect the larger and more polarizable ions to have greater binding energies.
Solvation effects
The nature of thesolvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
also determines the absolute and relative strength of the bonding. Most data on cation–π interaction is acquired in the gas phase
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a ...
, as the attraction is most pronounced in that case. Any intermediating solvent molecule will attenuate the effect, because the energy gained by the cation–π interaction is partially offset by the loss of solvation energy.
For a given cation–π adduct, the interaction energy decreases with increasing solvent polarity. This can be seen by the following calculated interaction energies of methylammonium and benzene in a variety of solvents.
Additionally, the trade-off between solvation and the cation–π effect results in a rearrangement of the order of interaction strength for a series of cations. While in the gas phase the most densely charged cations have the strongest cation–π interaction, these ions also have a high desolvation penalty.
This is demonstrated by the relative cation–π bond strengths in water for alkali metals:
:Nature of the π system
Quadrupole moment
Comparing the quadrupole moment of different arenes is a useful qualitative tool to predict trends in cation–π binding, since it roughly correlates with interaction strength. Arenes with larger quadrupole moments are generally better at binding cations. However, a quadrupole-ion model system cannot be used to quantitatively model cation–π interactions. Such models assumepoint charge
A point particle, ideal particle or point-like particle (often spelled pointlike particle) is an idealization of particles heavily used in physics. Its defining feature is that it lacks spatial extension; being dimensionless, it does not take u ...
s, and are therefore not valid given the short cation–π bond distance. In order to use electrostatics to predict energies, the full electrostatic potential surface must be considered, rather than just the quadrupole moment as a point charge.
Substituents on the aromatic ring
 The electronic properties of the
The electronic properties of the substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s also influence the strength of the attraction. Electron withdrawing groups (for example, cyano
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
−CN) weaken the interaction, while electron donating substituents (for example, amino −NH2) strengthen the cation–π binding. This relationship is illustrated quantitatively in the margin for several substituents.
The electronic trends in cation–π binding energy are not quite analogous to trends in aryl reactivity. Indeed, the effect of resonance participation by a substituent does not contribute substantively to cation–π binding, despite being very important in many chemical reactions with arenes. This was shown by the observation that cation–π interaction strength for a variety of substituted arenes correlates with the Hammett parameter. This parameter is meant to illustrate the inductive effects of functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
on an aryl ring.
The origin of substituent effects in cation–π interactions has often been attributed to polarization from electron donation or withdrawal into or out of the π system. This explanation makes intuitive sense, but subsequent studies have indicated that it is flawed. Recent computational work by Wheeler and Houk strongly indicate that the effect is primarily due to direct through-space interaction between the cation and the substituent dipole. In this study, calculations that modeled unsubstituted benzene plus interaction with a molecule of "H-X" situated where a substituent would be (corrected for extra hydrogen atoms) accounted for almost all of the cation–π binding trend. For very strong pi donors or acceptors, this model was not quite able account for the whole interaction; in these cases polarization may be a more significant factor.
Binding with heteroaromatic systems
Heterocycles are often activated towards cation–π binding when the lone pair on the heteroatom is in incorporated into the aromatic system (e.g.indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
, pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
). Conversely, when the lone pair does not contribute to aromaticity (e.g. pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
), the electronegativity of the heteroatom wins out and weakens the cation–π binding ability.
Since several classically "electron rich" heterocycles are poor donors when it comes to cation–π binding, one cannot predict cation–π trends based on heterocycle reactivity trends. Fortunately, the aforementioned subtleties are manifested in the electrostatic potential surfaces of relevant heterocycles.
cation–heterocycle interaction is not always a cation–π interaction; in some cases it is more favorable for the ion to be bound directly to a lone pair. For example, this is thought to be the case in pyridine-Na+ complexes.
Geometry
cation–π interactions have an approximate distance dependence of 1/rn where n<2. The interaction is less sensitive to distance than a simple ion-quadrupole interaction which has 1/r3 dependence. A study by Sherrill and coworkers probed the geometry of the interaction further, confirming that cation–π interactions are strongest when the cation is situated perpendicular to the plane of atoms (θ = 0 degrees in the image below). Variations from this geometry still exhibit a significant interaction which weakens as θ angle approaches 90 degrees. For off-axis interactions the preferred ϕ places the cation between two H atoms. Equilibrium bond distances also increase with off-axis angle. Energies where the cation is coplanar with the carbon ring aresaddle points
In mathematics, a saddle point or minimax point is a point on the surface of the graph of a function where the slopes (derivatives) in orthogonal directions are all zero (a critical point), but which is not a local extremum of the function. ...
on the potential energy surface
A potential energy surface (PES) or energy landscape describes the energy of a Physical system, system, especially a collection of atoms, in terms of certain Parameter, parameters, normally the positions of the atoms. The Surface (mathematics), ...
, which is consistent with the idea that interaction between a cation and the positive region of the quadrupole is not ideal.
Relative interaction strength
Theoretical calculations suggest the cation–π interaction is comparable to (and potentially stronger than) ammonium-carboxylatesalt bridge
In electrochemistry, a salt bridge or ion bridge is an essential laboratory device discovered over 100 years ago. It contains an electrolyte solution, typically an inert solution, used to connect the Redox, oxidation and reduction Half cell, ...
s in aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
media. Computed values below show that as solvent polarity increases, the strength of the cation–π complex decreases less dramatically. This trend can be rationalized by desolvation effects: salt bridge formation has a high desolvation penalty for both charged species whereas the cation–π complex would only pay a significant penalty for the cation.
In nature
Nature's building blocks contain aromatic moieties in high abundance. Recently, it has become clear that many structural features that were once thought to be purelyhydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
in nature are in fact engaging in cation–π interactions. The amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
side chains of phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of ...
, tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
, tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
, histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
, are capable of binding to cationic species such as charged amino acid side chains, metal ions, small-molecule neurotransmitters and pharmaceutical agents. In fact, macromolecular
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
binding sites that were hypothesized to include anionic groups (based on affinity for cations) have been found to consist of aromatic residues instead in multiple cases. Cation–π interactions can tune the pKa
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:H ...
of nitrogenous side-chains, increasing the abundance of the protonated form; this has implications for protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
structure and function. While less studied in this context, the DNA bases
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
are also able to participate in cation–π interactions.
Role in protein structure
Early evidence that cation–π interactions played a role inprotein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid ...
was the observation that in crystallographic data, aromatic side chains appear in close contact with nitrogen-containing side chains (which can exist as protonated, cationic species) with disproportionate frequency.
A study published in 1986 by Burley and Petsko looked at a diverse set of proteins and found that ~ 50% of aromatic residues Phe, Tyr, and Trp were within 6Å of amino groups. Furthermore, approximately 25% of nitrogen containing side chains Lys, Asn, Gln, and His were within van der Waals contact with aromatics and 50% of Arg in contact with multiple aromatic residues (2 on average).
Studies on larger data sets found similar trends, including some dramatic arrays of alternating stacks of cationic and aromatic side chains. In some cases the N-H hydrogens were aligned toward aromatic residues, and in others the cationic moiety was stacked above the π system. A particularly strong trend was found for close contacts between Arg and Trp. The guanidinium moiety of Arg in particular has a high propensity to be stacked on top of aromatic residues while also hydrogen-bonding with nearby oxygen atoms.
Molecular recognition and signaling
 An example of cation–π interactions in molecular recognition is seen in the
An example of cation–π interactions in molecular recognition is seen in the nicotinic acetylcholine receptor
Nicotinic acetylcholine receptors, or nAChRs, are Receptor (biochemistry), receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the c ...
(nAChR) which binds its endogenous ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
, acetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
(a positively charged molecule), via a cation–π interaction to the quaternary ammonium. The nAChR neuroreceptor is a well-studied ligand-gated ion channel
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as sodium, Na+, potassium, K+, calcium, Ca2+, and/or chloride, Cl− to ...
that opens upon acetylcholine binding. Acetylcholine receptors are therapeutic targets for a large host of neurological disorders, including Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become ...
, Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
, schizophrenia
Schizophrenia () is a mental disorder characterized variously by hallucinations (typically, Auditory hallucination#Schizophrenia, hearing voices), delusions, thought disorder, disorganized thinking and behavior, and Reduced affect display, f ...
, depression and autism
Autism, also known as autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by differences or difficulties in social communication and interaction, a preference for predictability and routine, sensory processing d ...
. Studies by Dougherty and coworkers confirmed that cation–π interactions are important for binding and activating nAChR by making specific structural variations to a key tryptophan residue and correlating activity results with cation–π binding ability.
The nAChR is especially important in binding nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As ...
in the brain, and plays a key role in nicotine addiction
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and '' Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used fo ...
. Nicotine has a similar pharmacophore to acetylcholine, especially when protonated. Strong evidence supports cation–π interactions being central to the ability of nicotine to selectively activate brain receptors without affecting muscle activity.
A further example is seen in the plant UV-B
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
sensing protein UVR8. Several tryptophan residues interact via cation–π interactions with arginine residues which in turn form salt bridges with acidic residues on a second copy of the protein. It has been proposed that absorption of a photon by the tryptophan residues disrupts this interaction and leads to dissociation of the protein dimer.
Cation–π binding is also thought to be important in cell-surface recognition
Enzyme catalysis
Cation–π interactions can catalyze chemical reactions by stabilizing buildup of positive charge intransition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s. This kind of effect is observed in enzymatic systems. For example, acetylcholine esterase
Acetylcholinesterase ( HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of ac ...
contains important aromatic groups that bind quaternary ammonium in its active site.
Polycyclization enzymes also rely on cation–π interactions. Since proton-triggered polycyclizations of squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
proceed through a (potentially concerted) cationic cascade, cation–π interactions are ideal for stabilizing this dispersed positive charge. The crystal structure of squalene-hopene cyclase shows that the active site is lined with aromatic residues.
In synthetic systems
Solid state structures
Cation–π interactions have been observed in the crystals of synthetic molecules as well. For example, Aoki and coworkers compared the solid state structures of Indole-3-acetic acid choline ester and an uncharged analogue. In the charged species, an intramolecular cation–π interaction with the indole is observed, as well as an interaction with the indole moiety of the neighboring molecule in the lattice. In the crystal of the isosteric neutral compound the same folding is not observed and there are no interactions between the ''tert''-butyl group and neighboring indoles.Supramolecular receptors
Some of the first studies on the cation–π interaction involved looking at the interactions of charged, nitrogenous molecules incyclophane
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit (typically a benzene ring) and a Catenation, chain that forms a bridge (chemical), bridge between two non-adjacent positions of the aromatic ring. More complex der ...
host–guest chemistry
In supramolecular chemistry, host–guest chemistry describes inclusion compound, complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bo ...
. It was found that even when anionic solubilizing groups were appended to aromatic host capsules, cationic guests preferred to associate with the π-system in many cases. The type of host shown to the right was also able to catalyze N-alkylation reactions to form cationic products.
More recently, cation–π centered substrate binding and catalysis has been implicated in supramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, ...
metal-ligand cluster catalyst systems developed by Raymond
Raymond is a male given name of Germanic origin. It was borrowed into English from French (older French spellings were Reimund and Raimund, whereas the modern English and French spellings are identical). It originated as the Germanic ᚱᚨᚷ� ...
and Bergman.
Use of π-π, CH-π, and π-cation interactions in supramolecular assembly
π-systems are important building blocks in supramolecular assembly because of their versatilenoncovalent interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The ...
with various functional groups. Particularly, π-π, CH-π, and π-cation interactions are widely used in supramolecular assembly and recognition.
 π-π interaction concerns the direct interactions between two &pi-systems; and cation–π interaction arises from the electrostatic interaction of a cation with the face of the π-system. Unlike these two interactions, the CH-π interaction arises mainly from charge transfer between the C-H orbital and the π-system.
A notable example of applying π-π interactions in supramolecular assembly is the synthesis of
π-π interaction concerns the direct interactions between two &pi-systems; and cation–π interaction arises from the electrostatic interaction of a cation with the face of the π-system. Unlike these two interactions, the CH-π interaction arises mainly from charge transfer between the C-H orbital and the π-system.
A notable example of applying π-π interactions in supramolecular assembly is the synthesis of catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
. The major challenge for the synthesis of catenane is to interlock molecules in a controlled fashion. Stoddart Stoddart is a surname. Notable people with the surname include:
*Alexander Stoddart, Alexander "Sandy" Stoddart (born 1959), Scottish sculptor
*Andrew Stoddart (1863–1915), English cricketer and rugby union player
*Archibald Peile Stoddart (1860� ...
and co-workers developed a series of systems utilizing the strong π-π interactions between electron-rich benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
derivatives and electron-poor pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is often ...
rings. atanene was synthesized by reacting bis(pyridinium) (A), bisparaphenylene-34-crown-10 (B), and 1, 4-bis(bromomethyl)benzene C (Fig. 2). The π-π interaction between A and B directed the formation of an interlocked template intermediate that was further cyclized by substitution reaction with compound C to generate the atenane product.

Organic synthesis and catalysis
Cation–π interactions have likely been important, though unnoticed, in a multitude of organic reactions historically. Recently, however, attention has been drawn to potential applications in catalyst design. In particular, noncovalent organocatalysts have been found to sometimes exhibit reactivity and selectivity trends that correlate with cation–π binding properties. A polycyclization developed by Jacobsen and coworkers shows a particularly strong cation–π effect using the catalyst shown below.Anion–π interaction
In many respects,anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
–π interaction is the opposite of cation–π interaction, although the underlying principles are identical. Significantly fewer examples are known to date. In order to attract a negative charge, the charge distribution of the π system has to be reversed. This is achieved by placing several strong electron withdrawing substituents along the π system (''e. g.'' hexafluorobenzene
Hexafluorobenzene, HFB or perfluorobenzene is an organofluorine compound with the chemical formula . In this derivative of benzene, all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although i ...
). The anion–π effect is advantageously exploited in chemical sensors for specific anions.
See also
*Stacking (chemistry)
In chemistry, stacking refers to superposition of molecules or atomic sheets owing to attractive interactions between these molecules or sheets.
Metal dichalcogenide compounds
Metal dichalcogenides have the formula ME2, where M = a transition me ...
* Salt bridge (protein)
In chemistry, a salt bridge is a combination of two non-covalent interactions: hydrogen bonding and ionic bonding (Figure 1). Ion pairing is one of the most important noncovalent forces in chemistry, in biological systems, in different materia ...
References
Sources
* . * {{DEFAULTSORT:Cation-pi interaction Chemical bonding