carboxylic on:
[Wikipedia]
[Google]
[Amazon]


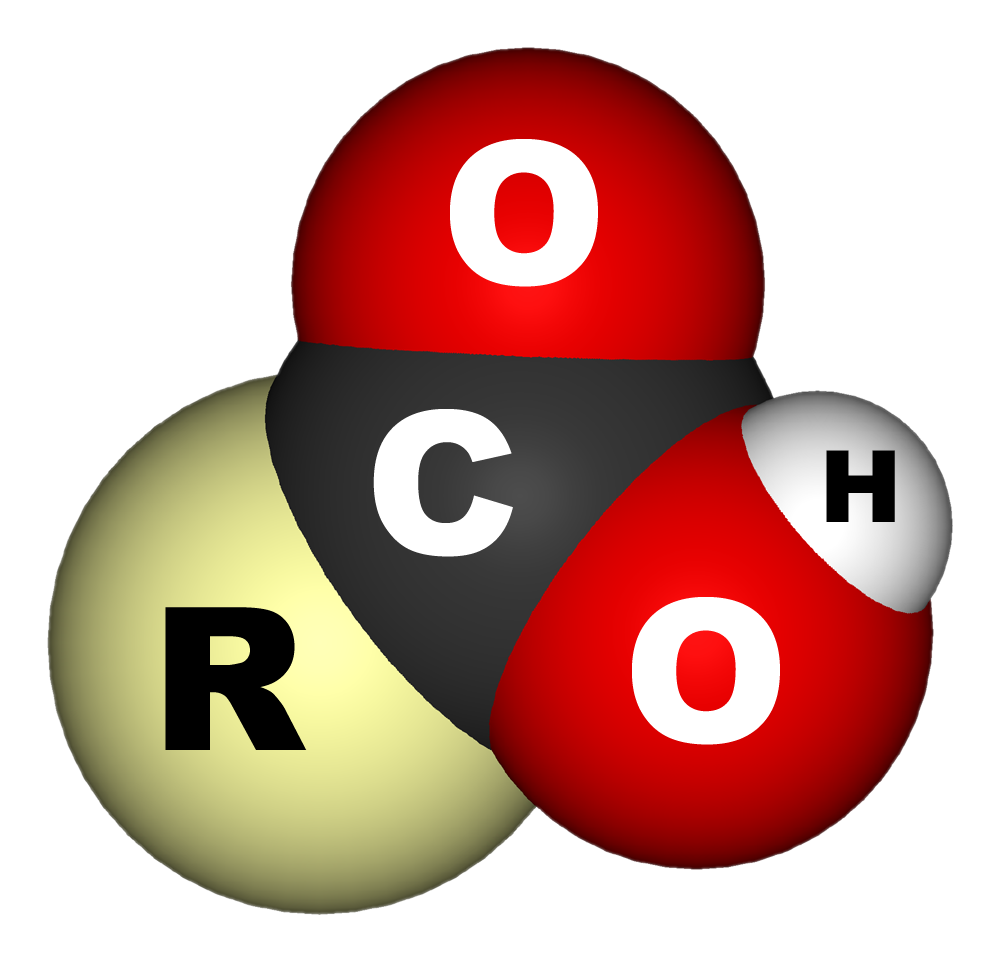 In
In



 Phosphorus(III) chloride (PCl3) and phosphorus(V) chloride (PCl5) will also convert carboxylic acids to acid chlorides, by a similar mechanism. One equivalent of PCl3 can react with three equivalents of acid, producing one equivalent of H3PO3, or phosphorus acid, in addition to the desired acid chloride. PCl5 reacts with carboxylic acids in a 1:1 ratio, and produces phosphorus(V) oxychloride (POCl3) and hydrogen chloride (HCl) as byproducts.
Phosphorus(III) chloride (PCl3) and phosphorus(V) chloride (PCl5) will also convert carboxylic acids to acid chlorides, by a similar mechanism. One equivalent of PCl3 can react with three equivalents of acid, producing one equivalent of H3PO3, or phosphorus acid, in addition to the desired acid chloride. PCl5 reacts with carboxylic acids in a 1:1 ratio, and produces phosphorus(V) oxychloride (POCl3) and hydrogen chloride (HCl) as byproducts.
– freeware for calculations, data analysis, simulation, and distribution diagram generation
PHC.
{{DEFAULTSORT:Carboxylic Acid Functional groups

 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
, alkenyl, aryl), or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s. Deprotonation of a carboxylic acid gives a carboxylate anion.
Examples and nomenclature
Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''.IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid
Butyric acid (; from , meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula . It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-met ...
() is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another parent structure, such as 2-carboxyfuran.
The carboxylate anion ( or ) of a carboxylic acid is usually named with the suffix ''-ate'', in keeping with the general pattern of ''-ic acid'' and ''-ate'' for a conjugate acid and its conjugate base, respectively. For example, the conjugate base of acetic acid is acetate.
Carbonic acid, which occurs in bicarbonate buffer systems in nature, is not generally classed as one of the carboxylic acids, despite it having a moiety that looks like a COOH group.
Physical properties
Solubility
Carboxylic acids are polar. Because they are both hydrogen-bond acceptors (the carbonyl ) and hydrogen-bond donors (the hydroxyl ), they also participate in hydrogen bonding. Together, the hydroxyl and carbonyl group form the functional group carboxyl. Carboxylic acids usually exist as dimers in nonpolar media due to their tendency to "self-associate". Smaller carboxylic acids (1 to 5 carbons) are soluble in water, whereas bigger carboxylic acids have limited solubility due to the increasing hydrophobic nature of the alkyl chain. These longer chain acids tend to be soluble in less-polar solvents such as ethers and alcohols. Aqueous sodium hydroxide and carboxylic acids, even hydrophobic ones, react to yield water-soluble sodium salts. For example, enanthic acid has a low solubility in water (0.2 g/L), but its sodium salt is very soluble in water. :
Boiling points
Carboxylic acids tend to have higher boiling points than water, because of their greater surface areas and their tendency to form stabilized dimers through hydrogen bonds. For boiling to occur, either the dimer bonds must be broken or the entire dimer arrangement must be vaporized, increasing the enthalpy of vaporization requirements significantly. :Acidity
Carboxylic acids are Brønsted–Lowry acids because they are proton (H+) donors. They are the most common type of organic acid. Carboxylic acids are typically weak acids, meaning that they only partially dissociate into cations and anions in neutralaqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
solution. For example, at room temperature, in a 1- molar solution of acetic acid, only 0.001% of the acid are dissociated (i.e. 10−5 moles out of 1 mol). Electron-withdrawing substituents such as trifluoromethyl () give stronger acids (the p''K''a of acetic acid is 4.76 whereas trifluoroacetic acid, with a trifluoromethyl substituent, has a p''K''a of 0.23). Electron-donating substituents give weaker acids (the p''K''a of formic acid is 3.75 whereas acetic acid, with a methyl substituent, has a p''K''a of 4.76)
Deprotonation of carboxylic acids gives carboxylate anions; these are resonance stabilized, because the negative charge is delocalized over the two oxygen atoms, increasing the stability of the anion. Each of the carbon–oxygen bonds in the carboxylate anion has a partial double-bond character. The carbonyl carbon's partial positive charge is also weakened by the −1/2 negative charges on the 2 oxygen atoms.
Odour
Carboxylic acids often have strong sour odours. Esters of carboxylic acids tend to have fruity, pleasant odours, and many are used in perfume.Characterization
Carboxylic acids are readily identified as such by infrared spectroscopy. They exhibit a sharp band associated with vibration of the C=O carbonyl bond (''ν''C=O) between 1680 and 1725 cm−1. A characteristic ''ν''O–H band appears as a broad peak in the 2500 to 3000 cm−1 region. By 1H NMR spectrometry, the hydroxyl hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.Occurrence and applications
Many carboxylic acids are produced industrially on a large scale. They are also frequently found in nature. Esters of fatty acids are the main components of lipids and polyamides of aminocarboxylic acids are the main components ofprotein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s.
Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, and food additives. Industrially important carboxylic acids include acetic acid (component of vinegar, precursor to solvents and coatings), acrylic and methacrylic acids (precursors to polymers, adhesives), adipic acid (polymers), citric acid (a flavor and preservative in food and beverages), ethylenediaminetetraacetic acid (chelating agent), fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s (coatings), maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis'' Cis–trans isomerism, isomer of butenedioic acid, ...
(polymers), propionic acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a ...
(food preservative), terephthalic acid (polymers). Important carboxylate salts are soaps.
Synthesis
Industrial routes
In general, industrial routes to carboxylic acids differ from those used on a smaller scale because they require specialized equipment. * Carbonylation of alcohols as illustrated by the Cativa process for the production of acetic acid. Formic acid is prepared by a different carbonylation pathway, also starting from methanol. * Oxidation of aldehydes with air using cobalt and manganese catalysts. The required aldehydes are readily obtained from alkenes by hydroformylation. * Oxidation of hydrocarbons using air. For simple alkanes, this method is inexpensive but not selective enough to be useful. Allylic and benzylic compounds undergo more selective oxidations. Alkyl groups on a benzene ring are oxidized to the carboxylic acid, regardless of its chain length. Benzoic acid from toluene, terephthalic acid from ''para''- xylene, and phthalic acid from ''ortho''- xylene are illustrative large-scale conversions. Acrylic acid is generated from propene. * Oxidation of ethene using silicotungstic acid catalyst. * Base-catalyzed dehydrogenation of alcohols. * Carbonylation coupled to the addition of water. This method is effective and versatile for alkenes that generate secondary and tertiary carbocations, e.g. isobutylene to pivalic acid. In the Koch reaction, the addition of water and carbon monoxide toalkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
or alkynes is catalyzed by strong acids. Hydrocarboxylations involve the simultaneous addition of water and CO. Such reactions are sometimes called " Reppe chemistry."
:
* Hydrolysis of triglycerides obtained from plant or animal oils. These methods of synthesizing some long-chain carboxylic acids are related to soap making.
* Fermentation of ethanol. This method is used in the production of vinegar.
* The Kolbe–Schmitt reaction provides a route to salicylic acid, precursor to aspirin.
Laboratory methods
Preparative methods for small scale reactions for research or for production of fine chemicals often employ expensive consumable reagents. * Oxidation of primary alcohols or aldehydes with strong oxidants such as potassium dichromate, Jones reagent, potassium permanganate, or sodium chlorite. The method is more suitable for laboratory conditions than the industrial use of air, which is "greener" because it yields less inorganic side products such as chromium or manganese oxides. * Oxidative cleavage of olefins by ozonolysis, potassium permanganate, or potassium dichromate. * Hydrolysis of nitriles, esters, or amides, usually with acid- or base-catalysis. * Carbonation of a Grignard reagent and organolithium reagents: : : * Halogenation followed by hydrolysis of methyl ketones in the haloform reaction * Base-catalyzed cleavage of non-enolizable ketones, especially aryl ketones: :Less-common reactions
Many reactions produce carboxylic acids but are used only in specific cases or are mainly of academic interest. * Disproportionation of an aldehyde in the Cannizzaro reaction * Rearrangement of diketones in the benzilic acid rearrangement * Involving the generation of benzoic acids are the von Richter reaction from nitrobenzenes and the Kolbe–Schmitt reaction fromphenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s.
Reactions
Acid-base reactions
Carboxylic acids react with bases to form carboxylate salts, in which thehydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
of the hydroxyl (–OH) group is replaced with a metal cation. For example, acetic acid found in vinegar reacts with sodium bicarbonate (baking soda) to form sodium acetate, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, and water:
:
Conversion to esters, amides, anhydrides
Widely practiced reactions convert carboxylic acids intoesters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
, amides, carboxylate salts, acid chlorides, and alcohols.
Their conversion to esters is widely used, e.g. in the production of polyesters. Likewise, carboxylic acids are converted into amides, but this conversion typically does not occur by direct reaction of the carboxylic acid and the amine. Instead esters are typical precursors to amides. The conversion of amino acids into peptides is a significant biochemical process that requires ATP.
Converting a carboxylic acid to an amide is possible, but not straightforward. Instead of acting as a nucleophile, an amine will react as a base in the presence of a carboxylic acid to give the ammonium carboxylate salt. Heating the salt to above 100 °C will drive off water and lead to the formation of the amide. This method of synthesizing amides is industrially important, and has laboratory applications as well.Wade 2010, pp. 964–965. In the presence of a strong acid catalyst, carboxylic acids can condense to form acid anhydrides. The condensation produces water, however, which can hydrolyze the anhydride back to the starting carboxylic acids. Thus, the formation of the anhydride via condensation is an equilibrium process.
Under acid-catalyzed conditions, carboxylic acids will react with alcohols to form esters via the Fischer esterification reaction, which is also an equilibrium process. Alternatively, diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
can be used to convert an acid to an ester. While esterification reactions with diazomethane often give quantitative yields, diazomethane is only useful for forming methyl esters.
Reduction
Like esters, most carboxylic acids can be reduced to alcohols byhydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, or using hydride transferring agents such as lithium aluminium hydride. Strong alkyl transferring agents, such as organolithium compounds but not Grignard reagents, will reduce carboxylic acids to ketones along with transfer of the alkyl group.
The Vilsmaier reagent (''N'',''N''-Dimethyl(chloromethylene)ammonium chloride; ) is a highly chemoselective agent for carboxylic acid reduction. It selectively activates the carboxylic acid to give the carboxymethyleneammonium salt, which can be reduced by a mild reductant like lithium tris(''t''-butoxy)aluminum hydride to afford an aldehyde in a one pot procedure. This procedure is known to tolerate reactive carbonyl functionalities such as ketone as well as moderately reactive ester, olefin, nitrile, and halide moieties.
Conversion to acyl halides
The hydroxyl group on carboxylic acids may be replaced with a chlorine atom using thionyl chloride to give acyl chlorides. In nature, carboxylic acids are converted to thioesters. Thionyl chloride can be used to convert carboxylic acids to their corresponding acyl chlorides. First, carboxylic acid 1 attacks thionyl chloride, and chloride ion leaves. The resulting oxonium ion 2 is activated towards nucleophilic attack and has a good leaving group, setting it apart from a normal carboxylic acid. In the next step, 2 is attacked by chloride ion to give tetrahedral intermediate 3, a chlorosulfite. The tetrahedral intermediate collapses with the loss ofsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
and chloride ion, giving protonated acyl chloride 4. Chloride ion can remove the proton on the carbonyl group, giving the acyl chloride 5 with a loss of HCl.
 Phosphorus(III) chloride (PCl3) and phosphorus(V) chloride (PCl5) will also convert carboxylic acids to acid chlorides, by a similar mechanism. One equivalent of PCl3 can react with three equivalents of acid, producing one equivalent of H3PO3, or phosphorus acid, in addition to the desired acid chloride. PCl5 reacts with carboxylic acids in a 1:1 ratio, and produces phosphorus(V) oxychloride (POCl3) and hydrogen chloride (HCl) as byproducts.
Phosphorus(III) chloride (PCl3) and phosphorus(V) chloride (PCl5) will also convert carboxylic acids to acid chlorides, by a similar mechanism. One equivalent of PCl3 can react with three equivalents of acid, producing one equivalent of H3PO3, or phosphorus acid, in addition to the desired acid chloride. PCl5 reacts with carboxylic acids in a 1:1 ratio, and produces phosphorus(V) oxychloride (POCl3) and hydrogen chloride (HCl) as byproducts.
Reactions with carbanion equivalents
Carboxylic acids react with Grignard reagents and organolithiums to form ketones. The first equivalent of nucleophile acts as a base and deprotonates the acid. A second equivalent will attack the carbonyl group to create a geminal alkoxide dianion, which is protonated upon workup to give the hydrate of a ketone. Because most ketone hydrates are unstable relative to their corresponding ketones, the equilibrium between the two is shifted heavily in favor of the ketone. For example, the equilibrium constant for the formation ofacetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
hydrate from acetone is only 0.002. The carboxylic group is the most acidic in organic compounds.
Specialized reactions
* As with all carbonyl compounds, the protons on the α-carbon are labile due to keto–enol tautomerization. Thus, the α-carbon is easily halogenated in the Hell–Volhard–Zelinsky halogenation. * The Schmidt reaction converts carboxylic acids to amines. * Carboxylic acids are decarboxylated in the Hunsdiecker reaction. * The Dakin–West reaction converts an amino acid to the corresponding amino ketone. * In the Barbier–Wieland degradation, a carboxylic acid on an aliphatic chain having a simple methylene bridge at the alpha position can have the chain shortened by one carbon. The inverse procedure is the Arndt–Eistert synthesis, where an acid is converted into acyl halide, which is then reacted withdiazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
to give one additional methylene in the aliphatic chain.
* Many acids undergo oxidative decarboxylation. Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s that catalyze these reactions are known as carboxylases ( EC 6.4.1) and decarboxylases (EC 4.1.1).
* Carboxylic acids are reduced to aldehydes via the ester and DIBAL, via the acid chloride in the Rosenmund reduction and via the thioester in the Fukuyama reduction.
* In ketonic decarboxylation carboxylic acids are converted to ketones.
* Organolithium reagents (>2 equiv) react with carboxylic acids to give a dilithium 1,1-diolate, a stable tetrahedral intermediate which decomposes to give a ketone upon acidic workup.
* The Kolbe electrolysis is an electrolytic, decarboxylative dimerization reaction. It gets rid of the carboxyl groups of two acid molecules, and joins the remaining fragments together.
Carboxyl radical
The carboxyl radical, •COOH, only exists briefly. The acid dissociation constant of •COOH has been measured using electron paramagnetic resonance spectroscopy.The value is p''K''a = −0.2 ± 0.1. The carboxyl group tends to dimerise to form oxalic acid.See also
* Acid anhydride * Acid chloride * Amide * Amino acid * Ester * List of carboxylic acids * Dicarboxylic acid * Pseudoacid * Thiocarboxy *Carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO2)
References
External links
* Carboxylic acids pH and titratio– freeware for calculations, data analysis, simulation, and distribution diagram generation
PHC.
{{DEFAULTSORT:Carboxylic Acid Functional groups