|
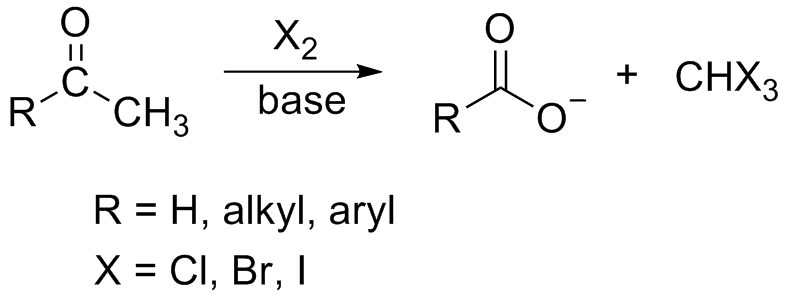
Haloform Reaction
In chemistry, the haloform reaction (also referred to as the Lieben haloform reaction) is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adolf Lieben
Adolf Lieben (3 December 1836 – 6 June 1914) was an Austrian Jewish chemist. He was born in Vienna the son of Ignatz Lieben. He studied at the University of Vienna, University of Heidelberg ( Ph.D. 1856 with Robert Wilhelm Bunsen), and Paris, and subsequently held the positions of privat-docent at the University of Vienna (1861), and professor in the universities of Palermo (1863), Turin (1867), and Prague (1871). From 1875 until his death he held the chair of general and pharmacological chemistry at the University of Vienna, and was a member of the Vienna Academy of Sciences. Publications Lieben has published many essays in '' Liebig's Annalen der Chemie'': *"Ueber die Einwirkung schwacher Affinitäten auf Aldehyde," 1861; *"Ueber das Iodbenzol," 1869; *"Ueber festes Benzoylchlorid," 1875; etc., *"Sitzungsberichte den Kaiserlichen Akademie der Wissenschaften in Wien" ("Untersuchungen über Milchzucker," "Einwirkung von Cyangas auf Aldehyde," "Ueber den Formaldehyd und de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform 1
Bromoform is an organic compound with the chemical formula . It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water (one part bromoform in 800 parts water) and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis Bromoform was discovered in 1832 by Löwig who distilled a mixture of bromal and potassium hydroxide, as analogous to preparation of chloroform from chloral. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetic Acid
Acetoacetic acid ( IUPAC name: 3-oxobutanoic acid, also known as acetonecarboxylic acid or diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta- keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid. Biochemistry Under typical physiological conditions, acetoacetic acid exists as its conjugate base, acetoacetate: : Unbound acetoacetate is primarily produced by liver mitochondria from its thioester with coenzyme A (CoA): : The acetoacetyl-CoA itself is formed by three routes: *3-hydroxy-3-methylglutaryl CoA releases acetyl CoA and acetoacetate: *: *Acetoacetyl-CoA can come from beta oxidation of butyryl-CoA: *: *Condensation of pair of acetyl CoA molecules as catalyzed by thiolase. *: In mammals, acetoacetate produced in the liver (along with the other two " ketone bodies") is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer . The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds. Properties Tautomerism The Keto–enol tautomerism, keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v molecular symmetry, symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when ''K''keto→enol is equal or greater than 1, the enol form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol (drug), alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. International Agency for Research on Cancer, The International Agency for Research on Cancer (IARC) has listed acetaldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor. Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins. Notably, it is not miscible with salt solutions and can be separated by adding sodium chloride in a process known as salting out. It forms an azeotrope with water, resulting in a boiling point of 80.37 °C and is characterized by its slightly bitter taste. Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm. Chemically, it can be oxidized to acetone or undergo various reactions to form compounds like isopropoxides or aluminium isopropoxide. As an isopropyl group linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloform Schritt 2
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Trihalomethanes with all the same halogen atoms are called haloforms. Many trihalomethanes find uses in industry as solvents or refrigerants. Some THMs are also environmental pollutants, and a few are considered carcinogenic. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene (TFE), precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trihalomethanes released to the environment break down faster than chlorofluorocarbons ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloral Hydrate
Chloral hydrate is a geminal diol with the formula . It was first used as a sedative and hypnotic in Germany in the 1870s. Over time it was replaced by safer and more effective alternatives but it remained in use in the United States until at least the 1970s. It sometimes finds usage as a laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water. Uses Hypnotic Chloral hydrate has not been approved by the FDA in the United States nor the EMA in the European Union for any medical indication and is on the FDA list of unapproved drugs that are still prescribed by clinicians. Usage of the drug as a sedative or hypnotic may carry some risk given the lack of clinical trials. However, chloral hydrate products, licensed for short-term management of severe insomnia, are available in the United Kingdom. Chloral hydrate was voluntarily removed from the market by all manufacturers in the United States in 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |