Acetaldehyde on:
[Wikipedia]
[Google]
[Amazon]
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the
 Like many other carbonyl compounds, acetaldehyde tautomerizes to give an
Like many other carbonyl compounds, acetaldehyde tautomerizes to give an
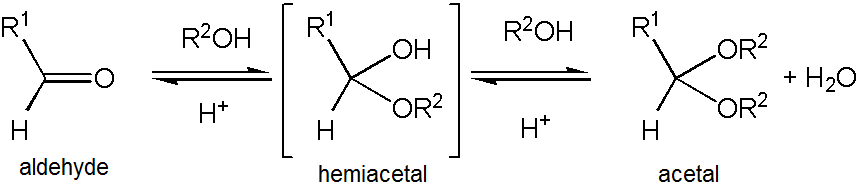 Acetaldehyde forms a stable
Acetaldehyde forms a stable '' )2 rather than referring to this specific compound — in fact, 1,1-diethoxyethane is also described as the diethyl acetal of acetaldehyde.
US Environmental Protection Agency In 1988 the
International Chemical Safety Card 0009
* * * Hal Kibbey
Indiana University Research and Creative Activity, Vol. 17 no. 3.
* [https://web.archive.org/web/20130601111815/http://www.inclusive-science-engineering.com/acetaldehyde-production-ethylene-oxidation-stage-process/ Acetaldehyde production process flow sheet by ethylene oxidation method] {{Authority control Alkanals Flavors Hepatotoxins IARC Group 2B carcinogens Organic compounds with 2 carbon atoms Recreational drug metabolites
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
, sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
by the liver enzyme alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
and is a contributing cause of hangover
A hangover is the experience of various unpleasant physiological and psychological effects usually following the consumption of alcohol (beverage), alcohol, such as wine, beer, and liquor. Hangovers can last for several hours or for more than ...
after alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by Enzyme inhibition, inhibiting the enzyme aldehyde dehydrogenase (specifically ALD ...
inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
. Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".
History
Acetaldehyde was first observed by the Swedish pharmacist/chemistCarl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybd ...
(1774); it was then investigated by the French chemists Antoine François, comte de Fourcroy and Louis Nicolas Vauquelin
Louis Nicolas Vauquelin FRS(For) HFRSE (; 16 May 1763 – 14 November 1829) was a French pharmacist and chemist. He was the discoverer of chromium and beryllium.
Early life
Vauquelin was born at Saint-André-d'Hébertot in Normandy, France, th ...
(1800), and the German chemists Johann Wolfgang Döbereiner (1821, 1822, 1832) and Justus von Liebig
Justus ''Freiherr'' von Liebig (12 May 1803 – 18 April 1873) was a Germans, German scientist who made major contributions to the theory, practice, and pedagogy of chemistry, as well as to agricultural and biology, biological chemistry; he is ...
(1835).
In 1835, Liebig named it "aldehyde", and in the middle of the century the name was altered to "acetaldehyde".
Production
In 2013, global production was about 438 thousand tons. Before 1962,ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
and acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
were the major sources of acetaldehyde. Since then, ethylene is the dominant feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials/Intermediate goods that are feedstock for future finishe ...
.
The main method of production is the oxidation of ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon double bonds).
Ethy ...
by the Wacker process, which involves oxidation of ethene using a homogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, i ...
palladium/copper catalyst system:
:
In the 1970s, the world capacity of the Wacker-Hoechst direct oxidation process exceeded 2 million tonnes annually.
Smaller quantities can be prepared by the partial oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of ethanol in an exothermic reaction. This process typically is conducted over a silver catalyst at about .
:
This method is one of the oldest routes for the industrial preparation of acetaldehyde.
Other methods
Hydration of acetylene
Prior to the Wacker process and the availability of cheap ethylene, acetaldehyde was produced by the hydration ofacetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
. This reaction is catalyzed by mercury(II) salts:
:
The mechanism involves the intermediacy of vinyl alcohol, which tautomerizes to acetaldehyde. The reaction is conducted at , and the acetaldehyde formed is separated from water and mercury and cooled to . In the wet oxidation process, iron(III) sulfate
Iron(III) sulfate or ferric sulfate (British English: sulphate instead of sulfate) is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulf ...
is used to reoxidize the mercury back to the mercury(II) salt. The resulting iron(II) sulfate
Iron(II) sulfate or ferrous sulfate (British English: sulphate instead of sulfate) denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7), but several values for ...
is oxidized in a separate reactor with nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
.
The enzyme Acetylene hydratase discovered in the strictly anaerobic bacterium '' Pelobacter acetylenicus'' can catalyze an analogous reaction without involving any compounds of mercury. However, it has thus far not been brought to any large-scale or commercial use.
Dehydrogenation of ethanol
Traditionally, acetaldehyde was produced by the partial dehydrogenation of ethanol: : In this endothermic process, ethanol vapor is passed at 260–290 °C over a copper-based catalyst. The process was once attractive because of the value of the hydrogen coproduct,Eckert, Marc ''et al.'' (2007) "Acetaldehyde" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim but in modern times is not economically viable.Hydroformylation of methanol
Thehydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
with catalysts like cobalt, nickel, or iron salts also produces acetaldehyde, although this process is of no industrial importance. Similarly noncompetitive, acetaldehyde arises from synthesis gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
with modest selectivity.
Reactions
Tautomerization to vinyl alcohol
enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
( vinyl alcohol; IUPAC name: ethenol):
: ∆''H''298,g = +42.7 kJ/mol
The equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
is 6 at room temperature, thus that the relative amount of the enol form in a sample of acetaldehyde is very small. At room temperature, acetaldehyde () is more stable than vinyl alcohol () by 42.7 kJ/mol: Overall the keto-enol tautomerization occurs slowly but is catalyzed by acids.
Photo-induced keto-enol tautomerization is viable under atmospheric
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere ...
or stratospheric conditions. This photo-tautomerization is relevant to the Earth's atmosphere, because vinyl alcohol is thought to be a precursor to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s in the atmosphere.
Addition and condensation reactions
Acetaldehyde is a common electrophile inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. In addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
s acetaldehyde is prochiral. It is used primarily as a source of the "" synthon in aldol reaction
The aldol reaction (aldol addition) is a Chemical reaction, reaction in organic chemistry that combines two Carbonyl group, carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might invol ...
s and related condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
s.. Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives. In one of the more spectacular addition reactions, formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
in the presence of calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
adds to MeCHO to give pentaerythritol, and formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.
Fundamentals
When dissolved in water, formic acid co ...
.
In a Strecker reaction, acetaldehyde condenses with cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
and ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
to give, after hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group sid ...
. Acetaldehyde can condense with amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s to yield imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s; for example, with cyclohexylamine to give ''N''- ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.
It is also a building block in the synthesis of heterocyclic compounds. In one example, it converts, upon treatment with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, to 5-ethyl-2-methylpyridine ("aldehyde-collidine").
Polymeric forms
Three molecules of acetaldehyde condense to form "paraldehyde
Paraldehyde is the cyclic trimer (chemistry), trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colo ...
", a cyclic trimer containing C-O single bonds. Similarly condensation of four molecules of acetaldehyde give the cyclic molecule metaldehyde
Metaldehyde is an organic compound with the chemical formula, formula (). It is used as a pesticide against slugs and snails. It is the cyclic tetramer of acetaldehyde.
Production and properties
Metaldehyde is flammable, toxic if ingested in large ...
. Paraldehyde can be produced in good yields, using a sulfuric acid catalyst. Metaldehyde is only obtained in a few percent yield and with cooling, often using HBr rather than as the catalyst. At in the presence of acid catalysts, polyacetaldehyde is produced. There are two stereomers of paraldehyde and four of metaldehyde.
The German chemist Valentin Hermann Weidenbusch (1821–1893) synthesized paraldehyde in 1848 by treating acetaldehyde with acid (either sulfuric or nitric acid) and cooling to . He found it quite remarkable that when paraldehyde was ''heated'' with a trace of the same acid, the reaction went the other way, recreating acetaldehyde.
Although vinyl alcohol is a polymeric form of acetaldehyde (), polyvinyl alcohol
Polyvinyl alcohol (PVOH, PVA, or PVAl) is a water- soluble synthetic polymer. It has the idealized formula H2CH(OH)sub>''n''. It is used in papermaking, textile warp sizing, as a thickener and emulsion stabilizer in polyvinyl acetate (PVAc) a ...
cannot be produced from acetaldehyde.
Acetal derivatives
 Acetaldehyde forms a stable
Acetaldehyde forms a stable acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
upon reaction with ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
under conditions that favor dehydration. The product, , is formally named 1,1-diethoxyethane but is commonly referred to as "acetal". This can cause confusion as "acetal" is more commonly used to describe compounds with the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s RCH(OR')2 or RR'C(ORPrecursor to vinylphosphonic acid
Acetaldehyde is a precursor to vinylphosphonic acid, which is used to make adhesives and ion conductive membranes. The synthesis sequence begins with a reaction with phosphorus trichloride: # # #Biochemistry
In theliver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
, the enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
oxidizes ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
into acetaldehyde, which is then further oxidized into harmless acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of to NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an ade ...
. In the brain, the enzyme catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting ...
is primarily responsible for oxidizing ethanol to acetaldehyde, and alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
plays a minor role. The last steps of alcoholic fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
in bacteria, plants, and yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
involve the conversion of pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
into acetaldehyde and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Many East Asian people
East Asian people (also East Asians) are the people from East Asia, which consists of China, Japan, Mongolia, North Korea, South Korea, and Taiwan. The total population of all countries within this region is estimated to be 1.677 billion and 21% ...
have an ALDH2
Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ''ALDH2'' gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the majo ...
mutation which makes them significantly less efficient at oxidizing acetaldehyde. On consuming alcohol, their bodies tend to accumulate excessive amounts of acetaldehyde, causing the so-called alcohol flush reaction. They develop a characteristic flush on the face and body, along with "nausea, headache and general physical discomfort". Ingestion of the drug disulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by Enzyme inhibition, inhibiting the enzyme aldehyde dehydrogenase (specifically ALD ...
, which inhibits ALDH2, leads to a similar reaction .
Uses
Traditionally, acetaldehyde was mainly used as a precursor to acetic acid. This application has declined because acetic acid is produced more efficiently from methanol by theMonsanto
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best-known product is Roundup, a glyphosate-based herbicide, developed ...
and Cativa processes. Acetaldehyde is an important precursor to pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
derivatives, pentaerythritol, and crotonaldehyde. Urea and acetaldehyde combine to give a useful resin
A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, predominantly terpenes. Commo ...
. Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
reacts with acetaldehyde to give ethylidene diacetate, a precursor to vinyl acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
Prod ...
, which is used to produce polyvinyl acetate.
The global market for acetaldehyde is declining. Demand has been impacted by changes in the production of plasticizer alcohols, which has shifted because ''n''-butyraldehyde is less often produced from acetaldehyde, instead being generated by hydroformylation of propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like o ...
. Likewise, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, once produced from acetaldehyde, is made predominantly by the lower-cost methanol carbonylation process. The impact on demand has led to increase in prices and thus slowdown in the market.China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
is the largest consumer of acetaldehyde in the world, accounting for almost half of global consumption in 2012. Major use has been the production of acetic acid. Other uses such as pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
s and pentaerythritol are expected to grow faster than acetic acid, but the volumes are not large enough to offset the decline in acetic acid. As a consequence, overall acetaldehyde consumption in China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
may grow slightly at 1.6% per year through 2018. Western Europe is the second-largest consumer of acetaldehyde worldwide, accounting for 20% of world consumption in 2012. As with China, the Western European acetaldehyde market is expected to increase only very slightly at 1% per year during 2012–2018. However, Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
could emerge as a potential consumer for acetaldehyde in the next five years due to newfound use in commercial production of butadiene. The supply of butadiene has been volatile in Japan and the rest of Asia. This should provide the much needed boost to the flat market, as of 2013.
Safety
Exposure limits
The threshold limit value is 25ppm (STEL/ceiling value) and the MAK (Maximum Workplace Concentration) is 50 ppm. At 50 ppm acetaldehyde, no irritation or local tissue damage in thenasal
Nasal is an adjective referring to the nose, part of human or animal anatomy. It may also be shorthand for the following uses in combination:
* With reference to the human nose:
** Nasal administration, a method of pharmaceutical drug delivery
* ...
mucosa
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It ...
is observed. When taken up by the organism, acetaldehyde is metabolized rapidly in the liver to acetic acid. Only a small proportion is exhaled unchanged. After intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutr ...
injection, the half-life in the blood is approximately 90 seconds.
Dangers
Toxicity
Many serious cases of acute intoxication have been recorded. Acetaldehyde naturally breaks down in the human body.Irritation
Acetaldehyde is an irritant of the skin, eyes, mucous membranes, throat, and respiratory tract. This occurs at concentrations as low as 1000 ppm. Symptoms of exposure to this compound includenausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. It can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat.
Over 30 d ...
, vomiting
Vomiting (also known as emesis, puking and throwing up) is the forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Vomiting can be the result of ailments like food poisoning, gastroenteritis, pre ...
, and headache
A headache, also known as cephalalgia, is the symptom of pain in the face, head, or neck. It can occur as a migraine, tension-type headache, or cluster headache. There is an increased risk of Depression (mood), depression in those with severe ...
. These symptoms may not happen immediately. The perception
Perception () is the organization, identification, and interpretation of sensory information in order to represent and understand the presented information or environment. All perception involves signals that go through the nervous syste ...
threshold for acetaldehyde in air is in the range between 0.07 and 0.25 ppm. At such concentrations, the fruit
In botany, a fruit is the seed-bearing structure in flowering plants (angiosperms) that is formed from the ovary after flowering.
Fruits are the means by which angiosperms disseminate their seeds. Edible fruits in particular have long propaga ...
y odor
An odor (American English) or odour ( Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive ...
of acetaldehyde is apparent. Conjunctiva
In the anatomy of the eye, the conjunctiva (: conjunctivae) is a thin mucous membrane that lines the inside of the eyelids and covers the sclera (the white of the eye). It is composed of non-keratinized, stratified squamous epithelium with gobl ...
l irritations have been observed after a 15-minute exposure to concentrations of 25 and 50 ppm, but transient conjunctivitis and irritation of the respiratory tract have been reported after exposure to 200 ppm acetaldehyde for 15 minutes.
Carcinogenicity
Acetaldehyde iscarcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
in humans.Chemical Summary For AcetaldehydeUS Environmental Protection Agency In 1988 the
International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC; ) is an intergovernmental agency forming part of the World Health Organization of the United Nations.
Its role is to conduct and coordinate research into the causes of cancer. It also cance ...
stated, "There is ''sufficient'' evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals." p3 In October 2009 the International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC; ) is an intergovernmental agency forming part of the World Health Organization of the United Nations.
Its role is to conduct and coordinate research into the causes of cancer. It also cance ...
updated the classification of acetaldehyde stating that acetaldehyde included in and generated endogenous
Endogeny, in biology, refers to the property of originating or developing from within an organism, tissue, or cell.
For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an ...
ly from alcoholic beverage
Drinks containing alcohol (drug), alcohol are typically divided into three classes—beers, wines, and Distilled beverage, spirits—with alcohol content typically between 3% and 50%. Drinks with less than 0.5% are sometimes considered Non-al ...
s is a Group I human carcinogen. In addition, acetaldehyde is damaging to DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and causes abnormal muscle development as it binds to proteins.
DNA crosslinks
Acetaldehyde induces DNA interstrand crosslinks, a form of DNA damage. These can be repaired by either of two replication-coupled DNA repair pathways. The first is referred to as the FA pathway, because it employs gene products defective in Fanconi's anemia patients. This repair pathway results in increased mutation frequency and altered mutational spectrum. The second repair pathway requires replication fork convergence, breakage of the acetaldehyde crosslink, translesion synthesis by a Y-family DNA polymerase and homologous recombination.Aggravating factors
Alzheimer's disease
People with a genetic deficiency for the enzyme responsible for the conversion of acetaldehyde intoacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
may have a greater risk of Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease and the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems wit ...
. "These results indicate that the ALDH2
Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ''ALDH2'' gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the majo ...
deficiency is a risk factor for LOAD ate-onset Alzheimer's disease..."
Genetic conditions
A study of 818 heavy drinkers found that those exposed to more acetaldehyde than normal through a genetic variant of the gene encoding for ADH1C, ADH1C*1, are at greater risk of developing cancers of theupper gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The tract is the largest of the body's systems, after the cardiovascular system. ...
and liver.
Disulfiram
The drugdisulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by Enzyme inhibition, inhibiting the enzyme aldehyde dehydrogenase (specifically ALD ...
(Antabuse) inhibits acetaldehyde dehydrogenase, an enzyme that oxidizes the compound into acetic acid. Metabolism of ethanol forms acetaldehyde before acetaldehyde dehydrogenase forms acetic acid, but with the enzyme inhibited, acetaldehyde accumulates. If one consumes ethanol while taking disulfiram, the hangover effect of ethanol is felt more rapidly and intensely ( disulfiram-alcohol reaction). As such, disulfiram is sometimes used as a deterrent for alcoholics wishing to stay sober.
Sources of exposure
Indoor air
Acetaldehyde is a potential contaminant in workplace, indoors, and ambient environments. Moreover, the majority of humans spend more than 90% of their time in indoor environments, increasing any exposure and the risk to human health. In a study inFrance
France, officially the French Republic, is a country located primarily in Western Europe. Overseas France, Its overseas regions and territories include French Guiana in South America, Saint Pierre and Miquelon in the Atlantic Ocean#North Atlan ...
, the mean indoor concentration of acetaldehydes measured in 16 homes was approximately seven times higher than the outside acetaldehyde concentration. The living room
In Western architecture, a living room, also called a lounge room (Australian English), lounge (British English), sitting room (British English), or drawing room, is a room for relaxing and socializing in a Dwelling, residential house or apa ...
had a mean of 18.1±17.5 μg m−3 and the bedroom
A bedroom or bedchamber is a room situated within a residential or accommodation unit characterized by its usage for sleeping. A typical Western world, western bedroom contains as bedroom furniture one or two beds, a clothes closet, and bedsid ...
was 18.2±16.9 μg m−3, whereas the outdoor air had a mean concentration of 2.3±2.6 μg m−3.
It has been concluded that volatile organic compounds (VOC) such as benzene, formaldehyde, acetaldehyde, toluene, and xylenes have to be considered priority pollutant
A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effect, or adversely affects the usefulness of a resource. These can be both naturally forming (i.e. minerals or extracted compounds like oi ...
s with respect to their health effects. It has been pointed that in renovated or completely new buildings, the VOCs concentration levels are often several orders of magnitude higher. The main sources of acetaldehydes in homes include building materials, laminate, PVC flooring, varnished wood flooring, and varnished cork/pine flooring (found in the varnish, not the wood). It is also found in plastics, oil-based and water-based paints, in composite wood ceilings, particle-board, plywood, treated pine wood, and laminated chipboard furniture.
Outdoor air
The use of acetaldehyde is widespread in different industries, and it may be released into waste water or the air during production, use, transportation and storage. Sources of acetaldehyde include fuel combustion emissions from stationary internal combustion engines and power plants that burn fossil fuels, wood, or trash, oil and gas extraction, refineries, cement kilns, lumber and wood mills and paper mills. Acetaldehyde is also present in automobile and diesel exhaust. As a result, acetaldehyde is "one of the most frequently found air toxics with cancer risk greater than one in a million".Tobacco smoke
Natural tobaccopolysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
, including cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
, have been shown to be the primary precursors making acetaldehyde a significant constituent of tobacco smoke. It has been demonstrated to have a synergistic
Synergy is an interaction or cooperation giving rise to a whole that is greater than the simple sum of its parts (i.e., a non-linear addition of force, energy, or effect). The term ''synergy'' comes from the Attic Greek word συνεργία ' f ...
effect with nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As ...
in rodent studies of addiction
Addiction is a neuropsychological disorder characterized by a persistent and intense urge to use a drug or engage in a behavior that produces natural reward, despite substantial harm and other negative consequences. Repetitive drug use can ...
. Acetaldehyde is also the most abundant carcinogen in tobacco smoke; it is dissolved into the saliva
Saliva (commonly referred as spit or drool) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which ...
while smoking.
Cannabis smoke
Acetaldehyde has been found in cannabis smoke. This finding emerged through the use of new chemical techniques that demonstrated the acetaldehyde present was causing DNA damage in laboratory settings.Alcohol consumption
Manymicrobes
A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from antiquity, with an early attestation in ...
produce acetaldehyde from ethanol, but they have a lower capacity to eliminate the acetaldehyde, which can lead to the accumulation of acetaldehyde in saliva, stomach acid, and intestinal contents. Fermented food and many alcoholic beverages can also contain significant amounts of acetaldehyde. Acetaldehyde, derived from mucosal or microbial oxidation of ethanol, tobacco smoke, and diet, appears to act as a cumulative carcinogen in the upper digestive tract of humans. According to European Commission's Scientific Committee on Consumer Safety's (SCCS) "Opinion on Acetaldehyde" (2012) the cosmetic products special risk limit is 5 mg/L and acetaldehyde should not be used in mouth-washing products.
Plastics
Acetaldehyde can be produced by the photo-oxidation ofpolyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in synthetic fibre, fibres for clothing, packaging, conta ...
(PET), via a Type II Norrish reaction.
Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/ odor threshold of around 20–40 ppb and can cause an off-taste in bottled water. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.
Candida overgrowth
The yeast '' Candida albicans'' in patients with potentially carcinogenic oral diseases has been shown to produce acetaldehyde in quantities sufficient to cause problems.See also
*Alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
* Disulfiram-like drug
* Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
* Paraldehyde
Paraldehyde is the cyclic trimer (chemistry), trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colo ...
* Wine fault
A wine fault is a sensory-associated (organoleptic) characteristic of a wine that is unpleasant, and may include elements of taste, smell, or appearance, elements that may arise from a "chemical or a microbial origin", where particular sensory expe ...
References
External links
International Chemical Safety Card 0009
* * * Hal Kibbey
Indiana University Research and Creative Activity, Vol. 17 no. 3.
* [https://web.archive.org/web/20130601111815/http://www.inclusive-science-engineering.com/acetaldehyde-production-ethylene-oxidation-stage-process/ Acetaldehyde production process flow sheet by ethylene oxidation method] {{Authority control Alkanals Flavors Hepatotoxins IARC Group 2B carcinogens Organic compounds with 2 carbon atoms Recreational drug metabolites