Carbenium on:
[Wikipedia]
[Google]
[Amazon]
 A carbenium ion is a positive ion with the structure RR′R″C+, that is, a
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a
 The tropylium ion is an
The tropylium ion is an
 An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in
An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in 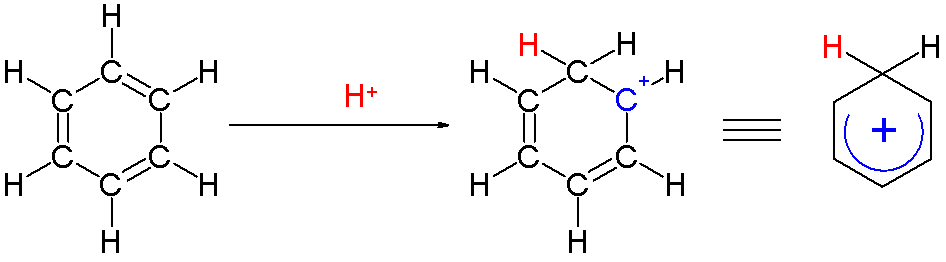 Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms via the π system, as depicted on the following
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms via the π system, as depicted on the following  Another contribution to the stability of arenium ions is the energy gain resulting from the strong bond between the benzene and the complexed electrophile.
The smallest arenium ion is protonated
Another contribution to the stability of arenium ions is the energy gain resulting from the strong bond between the benzene and the complexed electrophile.
The smallest arenium ion is protonated
chemical species
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the wa ...
with a trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of a ...
carbon that bears a +1 formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electrone ...
.
In older literature the name carbonium ion was used for this class, but now it refers exclusively to another family of carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
s, the carbonium ion
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms.
The ...
s, where the charged carbon is pentavalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of a ...
. The current definitions were proposed by the chemist George Andrew Olah
George Andrew Olah (born Oláh András György; May 22, 1927 – March 8, 2017) was a Hungarian-American chemist. His research involved the generation and reactivity of carbocations via superacids. For this research, Olah was awarded a Nobel Pr ...
in 1972, and are now widely accepted.
Carbenium ions are generally highly reactive due to having an incomplete octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 com ...
of electrons; however, certain carbenium ions, such as the tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.
Nomenclature
Carbenium ions are classified asprimary
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Work ...
, secondary
Secondary may refer to: Science and nature
* Secondary emission, of particles
** Secondary electrons, electrons generated as ionization products
* The secondary winding, or the electrical or electronic circuit connected to the secondary winding i ...
, or tertiary
Tertiary ( ) is a widely used but obsolete term for the geologic period from 66 million to 2.6 million years ago.
The period began with the demise of the non- avian dinosaurs in the Cretaceous–Paleogene extinction event, at the start ...
depending on whether the number of carbon atoms bonded to the ionized carbon is 1, 2, or 3. (Ions with zero carbons attached to the ionized carbon, such as methenium
In organic chemistry, methenium (also called methylium, carbenium, methyl cation, or protonated methylene) is a cation with the formula . It can be viewed as a methylene radical (:) with an added proton (), or as a methyl radical (•) with one ...
, , are usually included in the primary class).
Reactivity
Stability typically increases with the number ofalkyl groups
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
bonded to the charge-bearing carbon. Tertiary carbocations are more stable (and form more readily) than secondary carbocations, because they are stabilized by hyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electro ...
. Primary carbocations are highly unstable. Therefore, reactions such as the SN1 reaction and the E1 elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
normally do not occur if a primary carbenium would be formed.
However, a carbon doubly bonded with the ionized carbon can stabilize the ion by resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
. Such cations as the ''allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
'' cation, , and the ''benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a subst ...
'' cation, , are more stable than most other carbocations. Molecules which can form allyl or benzyl carbeniums are especially reactive. Carbenium ions can also be stabilized by heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecul ...
s.Hansjörg Grützmacher, Christina M. Marchand (1997), "Heteroatom stabilized carbenium ions", ''Coord. Chem. Rev.'', 163, 287–344.
Carbenium ions may undergo rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
s from less stable structures to equally stable or more stable ones with rate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the f ...
s in excess of 109 s−1. This fact complicates synthetic pathways to many compounds. For example, when pentan-3-ol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce 3-chloropentane and 2-chloropentane in a ratio of approximately 1:2.
Types of carbenium ions
Alkylium ions
Carbenium ions can be prepared directly fromalkane
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms tha ...
s by removing a hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
anion, , with a strong acid. For example, magic acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960 ...
, a mixture of antimony pentafluoride () and fluorosulfuric acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4 ...
(), turns isobutane into the trimethylcarbenium cation, .George A. Olah and Joachim Lukas (1967), "Stable Carbonium Ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion abstraction in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution". ''J. Am. Chem. Soc.'' 89 (18), 4739–4744
Aromatic carbenium ions
 The tropylium ion is an
The tropylium ion is an aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
species with the formula . Its name derives from the molecule tropine
Tropine is a derivative of tropane containing a hydroxyl group at the third carbon. It is also called 3-tropanol.
Tropine is a central building block of many chemicals active in the nervous system, including tropane alkaloids. Some of these comp ...
(itself named for the molecule atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically giv ...
). Salts of the tropylium cation can be stable, e.g. tropylium tetrafluoroborate
Tropylium tetrafluoroborate is an organic compound with the formula C7H7BF4. Containing the tropylium cation and the non-coordinating tetrafluoroborate counteranion, tropylium tetrafluoroborate is a rare example of a readily isolable carbocatio ...
. It can be made from cycloheptatriene
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical inte ...
(tropylidene) and bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
or phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moist ...
It is a planar, cyclic, heptagon
In geometry, a heptagon or septagon is a seven-sided polygon or 7-gon.
The heptagon is sometimes referred to as the septagon, using "sept-" (an elision of '' septua-'', a Latin-derived numerical prefix, rather than '' hepta-'', a Greek-derived n ...
al ion; it also has 6 π-electrons (4''n'' + 2, where ''n'' = 1), which fulfills Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ...
of aromaticity. It can coordinate as a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
to metal
A metal (from Greek μÎταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
.
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble salt from a reaction of cycloheptatriene and bromine. The structure was elucidated by Eggers Doering and Knox in 1954.
Another aromatic carbenium ion is the cyclopropenyl or cyclopropenium ion
The cyclopropenium ion is the cation with the formula . It has attracted attention as the smallest example of an aromatic cation. Its salts have been isolated, and many derivatives have been characterized by X-ray crystallography. The cation and so ...
, , obtained by Ronald Breslow and John T. Groves in 1970. Though less stable than the tropylium cation, this carbenium ion can also form salts at room temperature. Solutions of such salts were found by Breslow and Groves to have spectroscopic and chemical properties matching expectations for an aromatic carbenium ion.
Triphenylmethyl (trityl) cation
The triphenylcarbenium or triphenylmethyl cation, , is especially stable because the positive charge can be distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). It exists in the compoundstriphenylmethyl hexafluorophosphate
Triphenylmethyl (also triphenylcarbenium, trityl, or tritylium) hexafluorophosphate is an organic salt with the formula or , consisting of the triphenylmethyl cation and the hexafluorophosphate anion .
Triphenylmethyl hexafluorophosphate is a ...
, triphenylmethyl tetrafluoroborate , and triphenylmethyl perchlorate
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trit ...
.N. C. Deno, J. J. Jaruzelski, and Alan Schriesheim (1955) "Carbonium ions. I. An acidity function (''C''0) derived from arylcarbonium ion equilibria." ''J. Am. Chem. Soc.'', 77 (11), 3044–3051. Its derivatives include the triarylmethane dye
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
Families
Triarylmethane dyes can be grouped into families accordin ...
s.
Arenium ions
 An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in
An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. For historic reasons this complex is also called a ''Wheland intermediate'', or a ''σ-complex''.
: Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms via the π system, as depicted on the following
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms via the π system, as depicted on the following resonance structures
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ...
:
: Another contribution to the stability of arenium ions is the energy gain resulting from the strong bond between the benzene and the complexed electrophile.
The smallest arenium ion is protonated
Another contribution to the stability of arenium ions is the energy gain resulting from the strong bond between the benzene and the complexed electrophile.
The smallest arenium ion is protonated benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
, . The benzenium ion can be isolated as a stable compound when benzene is protonated by the carborane superacid
Carborane acids (X, Y, Z = H, Alk, F, Cl, Br, CF3) are a class of superacids, some of which are estimated to be at least one million times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (''H''0 ≤ –1 ...
, H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C. Bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest ...
s deduced from X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
are consistent with a cyclohexadienyl cation structure.
Acylium ions
An acylium ion is a cation with the formula RCO+. The structure is described as R−C≡O+ or R−=O. It is the synthetic and reactive equivalent of an acyl carbocation, but the actual structure has the oxygen and carbon linked by a triple bond. Such species are common reactive intermediates, for example, in the Friedel−Crafts acylations also in many otherorganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
s such as the Hayashi rearrangement The Hayashi rearrangement is the chemical reaction of ''ortho''-benzoylbenzoic acids catalyzed by sulfuric acid or phosphorus pentoxide.
This reaction proceeds through electrophilic acylium ion
In chemistry, an acyl group is a moiety derived b ...
. Salts containing acylium ions can be generated by removal of the halide from acyl halide
In organic chemistry, an acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen).
If the acid is a carboxylic acid (), the compound ...
s:
:RCOCl + SbCl5 → RCO+
The C–O distance in these cations is near 1.1 ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s, even shorter than that in carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. Acylium cations are characteristic fragments observed in EI-mass spectra
A mass spectrum is a histogram plot of intensity vs. ''mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for example ...
of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s.
See also
* Borenium ion *Nitrenium ion
A nitrenium ion (also called: aminylium ion or imidonium ion (obsolete)) in organic chemistry is a reactive intermediate based on nitrogen with both an electron lone pair and a positive charge and with two substituents (). Nitrenium ions are isoe ...
References
{{reflist Cations Reactive intermediates Carbocations