Butler–Volmer Equation on:
[Wikipedia]
[Google]
[Amazon]
In
 The Butler–Volmer equation is:
:
or in a more compact form:
:
where:
* : electrode
The Butler–Volmer equation is:
:
or in a more compact form:
:
where:
* : electrode
 The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and Faulkner and Newman and Thomas-Alyea. For a simple unimolecular, one-step
The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and Faulkner and Newman and Thomas-Alyea. For a simple unimolecular, one-step
electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, the Butler–Volmer equation (named after John Alfred Valentine Butler and Max Volmer
Max Volmer (; 3 May 1885 – 3 June 1965) was a German physical chemist, who made important contributions to materials science, photochemistry, and electrochemistry. Along with Weber, Volmer made early and pivotal contributions to the developme ...
), also known as Erdey-Grúz–Volmer equation, is one of the most fundamental relationships in electrochemical kinetics. It describes how the electrical current through an electrode depends on the voltage difference between the electrode and the bulk electrolyte for a simple, unimolecular redox reaction, considering that both a cathodic
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current ...
and an anodic reaction occur on the same electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
:
The Butler–Volmer equation
current density
In electromagnetism, current density is the amount of charge per unit time that flows through a unit area of a chosen cross section. The current density vector is defined as a vector whose magnitude is the electric current per cross-sectional ...
, A/m2 (defined as j ''= I/S'')
*: exchange current density, A/m2
* : electrode potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can c ...
, V
* : equilibrium potential, V
* : absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
, K
* : number of electrons involved in the electrode reaction
* : Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
* : universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature, temperature ...
*: so-called cathodic charge transfer coefficient, dimensionless
*: so-called anodic charge transfer coefficient, dimensionless
* : activation overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly r ...
(defined as ).
The right hand figure shows plots valid for .
The limiting cases
There are two limiting cases of the Butler–Volmer equation: * the low overpotential region (called "polarization resistance", i.e., when E ≈ Eeq), where the Butler–Volmer equation simplifies to: :; * the high overpotential region, where the Butler–Volmer equation simplifies to theTafel equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an Electrochemistry, electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical ...
. When , the first term dominates, and when , the second term dominates.
: for a cathodic reaction, when E << Eeq, or
: for an anodic reaction, when E >> Eeq
where and are constants (for a given reaction and temperature) and are called the Tafel equation constants. The theoretical values of the Tafel equation constants are different for the cathodic and anodic processes. However, the Tafel slope can be defined as:
:
where is the faradaic current, expressed as , being and the cathodic and anodic partial currents, respectively.
The extended Butler–Volmer equation
The more general form of the Butler–Volmer equation, applicable to the mass transfer-influenced conditions, can be written as: : where: * j is the current density, A/m2, * co and cr refer to the concentration of the species to be oxidized and to be reduced, respectively, * c(0,t) is the time-dependent concentration at the distance zero from the surface of the electrode. The above form simplifies to the conventional one (shown at the top of the article) when the concentration of the electroactive species at the surface is equal to that in the bulk. There are two rates which determine the current-voltage relationship for an electrode. First is the rate of the chemical reaction at the electrode, which consumes reactants and produces products. This is known as the ''charge transfer'' rate. The second is the rate at which reactants are provided, and products removed, from the electrode region by various processes including diffusion, migration, and convection. The latter is known as the '' mass-transfer'' rate . These two rates determine the concentrations of the reactants and products at the electrode, which are in turn determined by them. The slowest of these rates will determine the overall rate of the process. The simple Butler–Volmer equation assumes that the concentrations at the electrode are practically equal to the concentrations in the bulk electrolyte, allowing the current to be expressed as a function of potential only. In other words, it assumes that the mass transfer rate is much greater than the reaction rate, and that the reaction is dominated by the slower chemical reaction rate. Despite this limitation, the utility of the Butler–Volmer equation in electrochemistry is wide, and it is often considered to be "central in the phenomenological electrode kinetics".J. O'M. Bockris, A.K.N.Reddy, and M. Gamboa-Aldeco, "Modern Electrochemistry 2A. Fundamentals of Electrodics.", Second Edition, Kluwer Academic/Plenum Publishers, p.1083, 2000. The extended Butler–Volmer equation does not make this assumption, but rather takes the concentrations at the electrode as given, yielding a relationship in which the current is expressed as a function not only of potential, but of the given concentrations as well. The mass-transfer rate may be relatively small, but its only effect on the chemical reaction is through the altered (given) concentrations. In effect, the concentrations are a function of the potential as well. A full treatment, which yields the current as a function of potential only, will be expressed by the extended Butler–Volmer equation, but will require explicit inclusion of mass transfer effects in order to express the concentrations as functions of the potential.Derivation
General expression
 The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and Faulkner and Newman and Thomas-Alyea. For a simple unimolecular, one-step
The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and Faulkner and Newman and Thomas-Alyea. For a simple unimolecular, one-step reaction
Reaction may refer to a process or to a response to an action, event, or exposure.
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
* Chain reaction (disambiguation)
Biology and ...
of the form:
:O+ne− → R
The forward and backward reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
s (''vf'' and ''vb'') and, from Faraday's laws of electrolysis
Faraday's laws of electrolysis are quantitative relationships based on the electrochemical research published by Michael Faraday in 1833.
First law
Michael Faraday reported that the mass () of a substance deposited or liberated at an electrod ...
, the associated electrical current densities (''j''), may be written as:
:
:
where ''kf'' and ''kb'' are the reaction rate constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.
For a reaction between ...
s, with units of frequency (1/time) and ''co'' and ''cr'' are the surface concentrations (mol/area) of the oxidized and reduced molecules, respectively (written as ''co(0,t)'' and ''cr(0,t)'' in the previous section). The net rate of reaction ''v'' and net current density ''j'' are then:
:
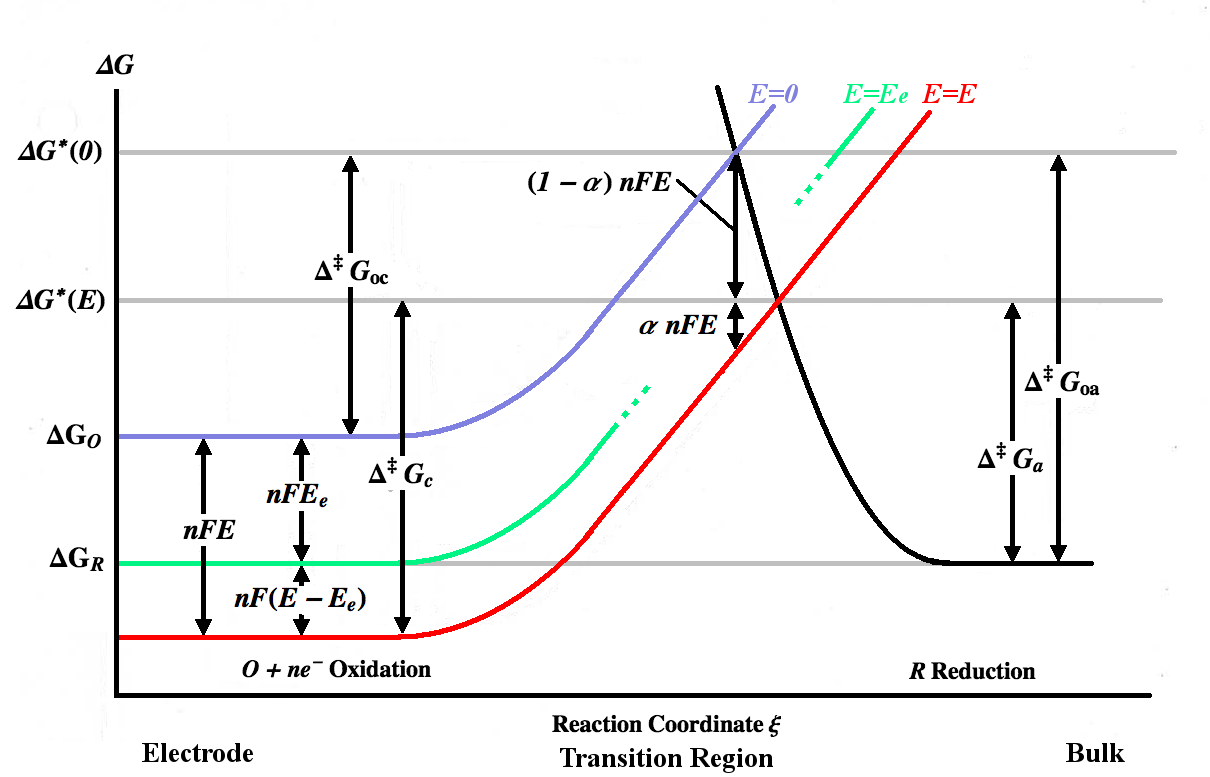
The figure above plots various Gibbs energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a ther ...
curves as a function of the reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate chosen to represent progress along a reaction pathway. Where possible it is usually a geometric parameter that changes during the conversion of one or more molecular e ...
ξ. The reaction coordinate is roughly a measure of distance, with the body of the electrode being on the left, the bulk solution being on the right. The blue energy curve shows the increase in Gibbs energy for an oxidized molecule as it moves closer to the surface of the electrode when no potential is applied. The black energy curve shows the increase in Gibbs energy as a reduced molecule moves closer to the electrode. The two energy curves intersect at . Applying a potential ''E'' to the electrode will move the energy curve downward (to the red curve) by ''nFE'' and the intersection point will move to . and are the activation energies (energy barriers) to be overcome by the oxidized and reduced species respectively for a general ''E'', while and are the activation energies for ''E=0''.
Assume that the rate constants are well approximated by an Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 188 ...
,
: