Bromine Cycle on:
[Wikipedia]
[Google]
[Amazon]
 The bromine cycle is a
The bromine cycle is a
 Bromine is present naturally as bromide salts in
Bromine is present naturally as bromide salts in
Br + O3 -> BrO + O2
BrO + hv -> 2Br + O3
BrO + HO2 -> HBrO + O2
BrO + NO -> Br + NO2
The bromine explosion reaction seen below is of concern as two Br radicals are produced through these reactions from one starting Br radical. This increases the amount of Br available to react with ozone. This reaction converts HOx to BrOx.
Br + HO2 + Br^-/HBr (surface) + hv -> 2 Br + OH^-/H2O
Bromine cycling and interactions with ozone are dependent on VOC and NOx concentrations. Research and models suggest bromine contributes to 5-15% of
 The bromine cycle is a
The bromine cycle is a biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
of bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
through the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
, and hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
. Bromine has natural and anthropogenic sources, impacting each sphere as bromine is stored, released, or taken up. Ozone depletion and health hazards to humans, animals, and plants are effects of bromine throughout the environment.
Sources
Natural sources
 Bromine is present naturally as bromide salts in
Bromine is present naturally as bromide salts in evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as oce ...
deposits. Bromine is also present in soils and marine algae
Marine is an adjective meaning of or pertaining to the sea or ocean.
Marine or marines may refer to:
Ocean
* Maritime (disambiguation)
* Marine art
* Marine biology
* Marine current power
* Marine debris
* Marine energy
* Marine habitats
* M ...
that synthesize organic bromine compounds. Other natural sources of bromine come from polar ice and snow, salt lakes, and volcanoes.
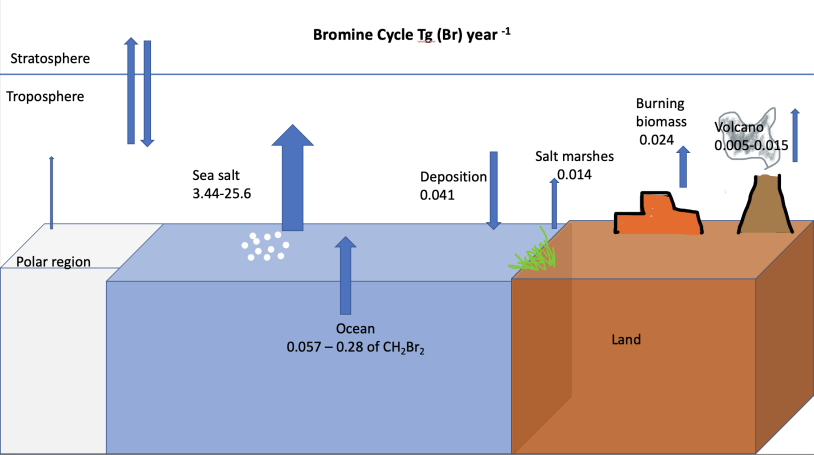
The primary natural source of bromine to the atmosphere is sea spray
Sea spray consists of aerosol particles formed from the ocean, primarily by ejection into Earth's atmosphere through bursting bubbles at the air-sea interface Sea spray contains both organic matter and inorganic salts that form sea salt aeroso ...
aerosols. Oceans contain small amounts of bromine due to waves perturbing gas bubbles containing bromine, as well as marine biota producing bromine containing compounds. The lifetime of bromine from sea spray aerosols is determined by the time it takes for photolysis to release bromine from sea spray aerosols to bromine in the gas phase. Additionally, the lifetime of bromine depends on the rate of deposition of sea spray aerosols, which can shorten the lifetime of bromine in the atmosphere. Smaller fluxes originate from volcanic emissions, releasing HBr and BrO during passive degassing and explosion events, and biomass burning.
The primary atmospheric sinks are sea spray deposition and photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
, which release gaseous bromine.
Anthropogenic sources and uses
Bromine is used in flame retardants, pesticides mostly in the form of CHBr3, pharmaceuticals, mining and oil drilling, lighter and gasoline fuels, antiknocking agents, and water purification methods. The organic form of this element is used as flame retardants commercially and in pesticides. These sources are important as they have been identified to increase the depletion of the stratospheric ozone layer. Some countries use bromine to treat drinking water, similar to chlorination. Bromine is also present as impurities emitted fromcooling tower
A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream, to a lower temperature. Cooling towers may either use the evaporation of water to remove heat and cool the ...
s.
Health Hazards
Bromine in high concentrations can had adverse effects on human health, as well as environmental systems. High concentrations of bromine can limit plant growth. By-products from using bromine to treat water have been linked to increased cancer risk. Some bromine containing compounds, such as those used for pesticides in agriculture, have been determined to cause health risks to the liver, stomach, kidneys, and organs associated with reproduction. Compounds associated with flame retardants are considered toxic and persistent in the environment, as they can accumulate throughout food chains. Exposure to bromine containing compounds can achieved through inhalation, ingestion, and absorption through the skin. Repeated exposure is linked to cancer for some bromine containing compounds, while others are associated with nervous system failure or corrosion of the skin. However, the Br- ion is not considered a concern to human health at typical environmental concentrations.Bromine in the atmosphere
Bromine is released into the atmosphere through a variety of sources. Once bromine is in a gaseous state in the atmosphere, photolysis and chemical reactions can break apart compounds containing bromine or form new bromine containing compounds. Bromine can quickly cycle between gas and particle phase throughout the atmosphere. Due to the quick cycling the main loss mechanisms include dry and wet deposition. Deposition allows bromine to be taken out of the atmosphere and move to the lithosphere and hydrosphere. Background concentrations of bromine containing compounds CHBr3 and CH2Br2 are 1-2 ppt.Tropospheric O3 depletion
Bromine is important to the ozone balance of the atmosphere. Bromine can react with ozone to produce BrO and O2. BrO has the ability to photolyze back to Br and O3 or BrO can react to HO2 to form HOBr. HOBr readily cycles to the aqueous phase. In high NOx areas, BrO can react with NO to produce Br and NO2. This reaction releases Br back into the atmosphere where it can continue to destroy ozone. These reactions rely on sunlight, so ozone is depleted at a greater rate in the summer months.tropospheric
The troposphere is the lowest layer of the atmosphere of Earth. It contains 80% of the total mass of the planetary atmosphere and 99% of the total mass of water vapor and aerosols, and is where most weather phenomena occur. From the planetary s ...
ozone layer losses. These reactions deplete the ozone within the atmosphere, as well as alter the oxidation potential of atmosphere.
Stratospheric O3 depletion
Winter sea ice is a significant atmospheric contribution of bromine, especially as polar regions were a significant location to understand halogen influence in the troposphere. Organic bromine gases such as CH3Br, CH2Br2, CH2IBr are emitted by microorganisms in sea ice and snow at ten-fold higher rates than from other environments.{{Cite book , last1=Seinfeld , first1=John H. , title=Atmospheric chemistry and physics: from air pollution to climate change , last2=Pandis , first2=Spyros N. , date=2016 , publisher=John Wiley & Sons , isbn=978-1-119-22116-6 , edition=Third , location=Hoboken, New Jersey Additionally, these gases are a source of bromine in the stratosphere. In polar areas, decreasing sea ice due to melting releases bromine and at the Arctic and Antarcticboundary layer
In physics and fluid mechanics, a boundary layer is the thin layer of fluid in the immediate vicinity of a Boundary (thermodynamic), bounding surface formed by the fluid flowing along the surface. The fluid's interaction with the wall induces ...
. Polar Stratospheric Clouds (PSC) also play a role in the availability of bromine in the stratosphere. PSCs can act as surfaces for reactions to occur on, allowing for bromine to become activated Br radicals, which are reactive with other compounds, including ozone. Br radicals in the stratosphere react with ozone to form BrO, which can further react with HOx, ClOx, or O, destroying stratospheric ozone.
Bromine in soils
Bromine can be stored within the soils and vegetation until it is released into the atmosphere or washed into rivers and oceans. Bacteria release Br- into the water in the soils and sediments through respiration, however, carbon content of the soil can influence the concentration of Br-. Limited adsorption in soils and sediments mean Br- is able to move thought soils and sediments easily. Since Br- is very mobile, Br- can easily be washed away with groundwater. Landfills can hold sources of bromine, which can be transported through runoff from precipitation and liquids within the landfill. Runoff from landfills can penetrate soils, which eventually can get into rivers, oceans, and other water sources. In efforts to remove bromine containing compounds in the soils, various methods including soil washing, biological mediation, thermal degradation, and other emerging methods have been tested and utilized.Bromine in the oceans
As seen above, Br- can be stored in soils, however, the concentration is much lower than the amount of Br- in the oceans. The ocean provides a large reservoir for Br- ions in the aqueous phase. The major reason for the difference between land and ocean storage is the deposition of Br- onto sea salt aerosols. Organisms in the oceans utilize bromine containing compounds to help defend against predation and grazing. While some organisms utilize bromine, removal of bromine containing compounds in the oceans has occurred through three types of methods: membrane, electrochemical, and adsorption methods.References