Azomethine Ylide on:
[Wikipedia]
[Google]
[Amazon]
 Azomethine ylides are nitrogen-based
Azomethine ylides are nitrogen-based
 Three different ylide shapes are possible, each leading to different stereochemistry in the products of 1,3-dipolar cycloaddition reactions. W-shaped, U-shaped, and S-shaped ylides are possible. The W- and U-shaped ylides, in which the R substituents are on the same side, result in ''syn'' cycloaddition products, whereas S-shaped ylides result in ''anti'' products. In the examples below, where the R3 substituent ends up in the product depends on the substituent's steric and electronic nature (see regioselectivity of 1,3 dipolar cycloadditions). The stereochemistry of R1 and R2 in the cycloaddition product is derived from the dipole. The stereochemistry of R3 is derived from the dipolarophile—if the dipolarophile is more than mono-substituted (and prochiral), up to four new stereocenters can result in the product.
Three different ylide shapes are possible, each leading to different stereochemistry in the products of 1,3-dipolar cycloaddition reactions. W-shaped, U-shaped, and S-shaped ylides are possible. The W- and U-shaped ylides, in which the R substituents are on the same side, result in ''syn'' cycloaddition products, whereas S-shaped ylides result in ''anti'' products. In the examples below, where the R3 substituent ends up in the product depends on the substituent's steric and electronic nature (see regioselectivity of 1,3 dipolar cycloadditions). The stereochemistry of R1 and R2 in the cycloaddition product is derived from the dipole. The stereochemistry of R3 is derived from the dipolarophile—if the dipolarophile is more than mono-substituted (and prochiral), up to four new stereocenters can result in the product.

 In this ring opening reaction, there is an issue of torquoselectivity. Electronegative substituents prefer to rotate outwards, to the same side as the R substituent on the nitrogen, whereas electropositive substituents prefer to rotate inwards.
Note that with aziridines, ring opening can result in a different 1,3-dipole, in which a C–N bond (rather than the C–C bond) breaks.
In this ring opening reaction, there is an issue of torquoselectivity. Electronegative substituents prefer to rotate outwards, to the same side as the R substituent on the nitrogen, whereas electropositive substituents prefer to rotate inwards.
Note that with aziridines, ring opening can result in a different 1,3-dipole, in which a C–N bond (rather than the C–C bond) breaks.
 One of the easiest methods of forming azomethine ylides is by condensation of an aldehyde with an amine. If the amine contains an electron-withdrawing group on the alpha carbon, such as an ester, the deprotonation occurs readily. A possible disadvantage of using this method is that the ester ends up in the cycloaddition product. An alternative is to use a
One of the easiest methods of forming azomethine ylides is by condensation of an aldehyde with an amine. If the amine contains an electron-withdrawing group on the alpha carbon, such as an ester, the deprotonation occurs readily. A possible disadvantage of using this method is that the ester ends up in the cycloaddition product. An alternative is to use a
 Azomethine ylides can also be formed directly by deprotonation of iminiums.
Azomethine ylides can also be formed directly by deprotonation of iminiums.
 The metal reagents used in this reaction include lithium bromide and silver acetate. In this method, the metal coordinates to the nitrogen in order to activate the substrate for deprotonation. Another way to form azomethine ylides from imines is by
The metal reagents used in this reaction include lithium bromide and silver acetate. In this method, the metal coordinates to the nitrogen in order to activate the substrate for deprotonation. Another way to form azomethine ylides from imines is by
 As with other cycloaddition reactions of a
As with other cycloaddition reactions of a 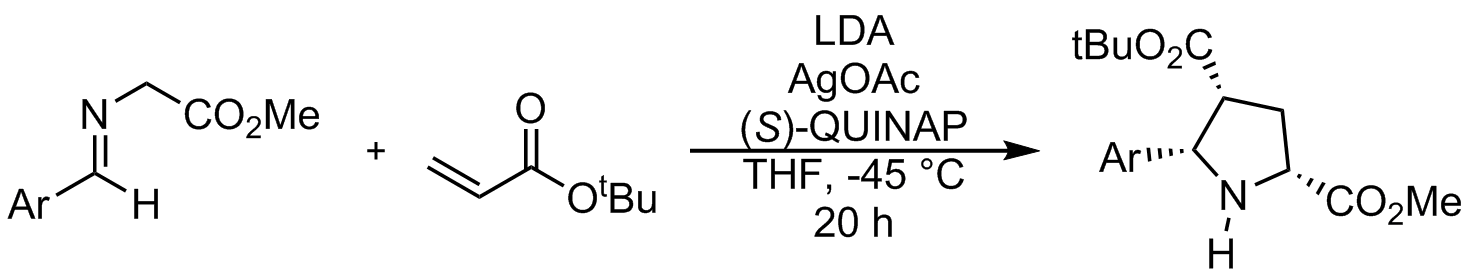 1,3-Dipolar cycloaddition reactions of azomethine ylides commonly use alkenes or
1,3-Dipolar cycloaddition reactions of azomethine ylides commonly use alkenes or
 The compounds resulting from this type of electrocyclization have been used as dienes in
The compounds resulting from this type of electrocyclization have been used as dienes in
 A cycloaddition of an azomethine ylide with an unactivated alkene was used in total synthesis of martinellic acid. The cycloaddition step formed two rings, including a
A cycloaddition of an azomethine ylide with an unactivated alkene was used in total synthesis of martinellic acid. The cycloaddition step formed two rings, including a
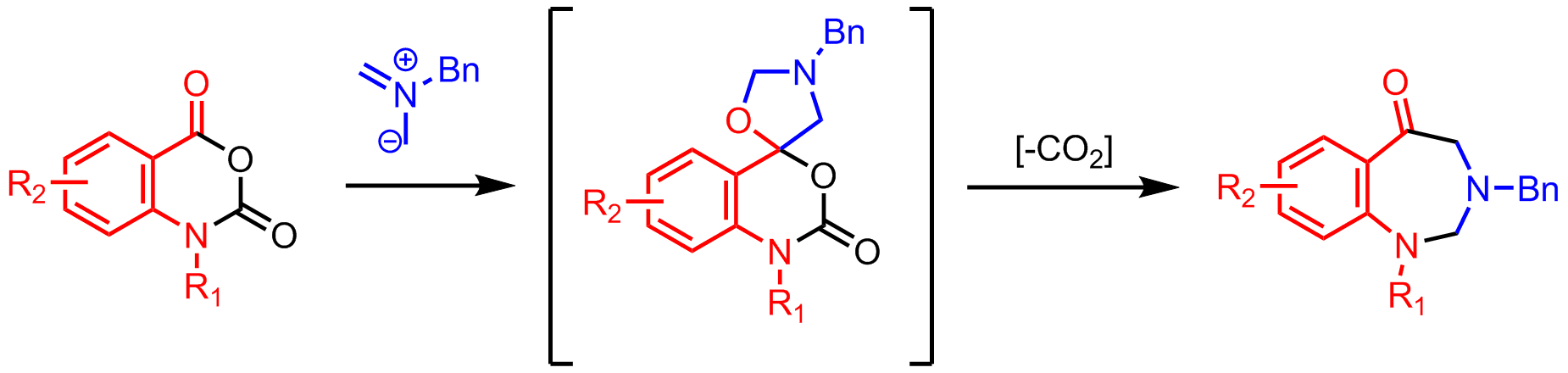 Cyclization of an azomethine ylide with a carbonyl affords a spirocyclic oxazolidine, which loses CO2 to form a seven-membered ring. These high-utility
Cyclization of an azomethine ylide with a carbonyl affords a spirocyclic oxazolidine, which loses CO2 to form a seven-membered ring. These high-utility
1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions.
The dipole has at least one resonance st ...
s, consisting of an iminium ion next to a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
. They are used in 1,3-dipolar cycloaddition reactions to form five-membered heterocycles, including pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
s and pyrrolines. These reactions are highly stereo- and regioselective, and have the potential to form four new contiguous stereocenters. Azomethine ylides thus have high utility in total synthesis, and formation of chiral ligands and pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and rel ...
. Azomethine ylides can be generated from many sources, including aziridines, imines, and iminiums. They are often generated ''in situ'', and immediately reacted with dipolarophiles.
Structure
The resonance structures below show the1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions.
The dipole has at least one resonance st ...
contribution, in which the two carbon atoms adjacent to the nitrogen have a negative or positive charge. The most common representation of azomethine ylides is that in which the nitrogen is positively charged, and the negative charge is shared between the two carbon atoms. The relative contributions of the different resonance structures depend on the substituents on each atom. The carbon containing electron-withdrawing substituents will have a more partial negative charge, due to the ability of the nearby electron-withdrawing group to stabilize the negative charge.
 Three different ylide shapes are possible, each leading to different stereochemistry in the products of 1,3-dipolar cycloaddition reactions. W-shaped, U-shaped, and S-shaped ylides are possible. The W- and U-shaped ylides, in which the R substituents are on the same side, result in ''syn'' cycloaddition products, whereas S-shaped ylides result in ''anti'' products. In the examples below, where the R3 substituent ends up in the product depends on the substituent's steric and electronic nature (see regioselectivity of 1,3 dipolar cycloadditions). The stereochemistry of R1 and R2 in the cycloaddition product is derived from the dipole. The stereochemistry of R3 is derived from the dipolarophile—if the dipolarophile is more than mono-substituted (and prochiral), up to four new stereocenters can result in the product.
Three different ylide shapes are possible, each leading to different stereochemistry in the products of 1,3-dipolar cycloaddition reactions. W-shaped, U-shaped, and S-shaped ylides are possible. The W- and U-shaped ylides, in which the R substituents are on the same side, result in ''syn'' cycloaddition products, whereas S-shaped ylides result in ''anti'' products. In the examples below, where the R3 substituent ends up in the product depends on the substituent's steric and electronic nature (see regioselectivity of 1,3 dipolar cycloadditions). The stereochemistry of R1 and R2 in the cycloaddition product is derived from the dipole. The stereochemistry of R3 is derived from the dipolarophile—if the dipolarophile is more than mono-substituted (and prochiral), up to four new stereocenters can result in the product.

Generation
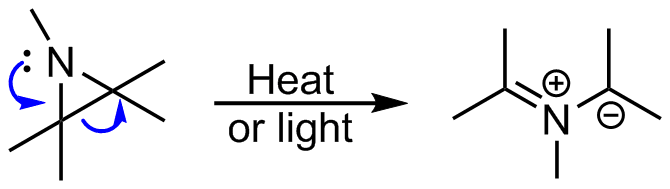
From aziridines
Azomethine ylides can be generated from ring opening of aziridines. In accordance with the Woodward–Hoffmann rules, the thermal four-electron ring opening proceeds via a conrotatory process, whereas the photochemical reaction is disrotatory. In this ring opening reaction, there is an issue of torquoselectivity. Electronegative substituents prefer to rotate outwards, to the same side as the R substituent on the nitrogen, whereas electropositive substituents prefer to rotate inwards.
Note that with aziridines, ring opening can result in a different 1,3-dipole, in which a C–N bond (rather than the C–C bond) breaks.
In this ring opening reaction, there is an issue of torquoselectivity. Electronegative substituents prefer to rotate outwards, to the same side as the R substituent on the nitrogen, whereas electropositive substituents prefer to rotate inwards.
Note that with aziridines, ring opening can result in a different 1,3-dipole, in which a C–N bond (rather than the C–C bond) breaks.
By condensation of aldehyde with amine
 One of the easiest methods of forming azomethine ylides is by condensation of an aldehyde with an amine. If the amine contains an electron-withdrawing group on the alpha carbon, such as an ester, the deprotonation occurs readily. A possible disadvantage of using this method is that the ester ends up in the cycloaddition product. An alternative is to use a
One of the easiest methods of forming azomethine ylides is by condensation of an aldehyde with an amine. If the amine contains an electron-withdrawing group on the alpha carbon, such as an ester, the deprotonation occurs readily. A possible disadvantage of using this method is that the ester ends up in the cycloaddition product. An alternative is to use a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, which can easily be removed during the cycloaddition process by decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
.
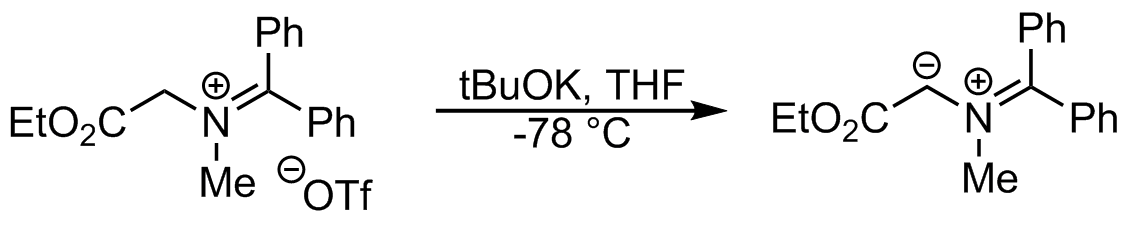
From imines and iminiums
 Azomethine ylides can also be formed directly by deprotonation of iminiums.
Azomethine ylides can also be formed directly by deprotonation of iminiums.
By ''N''-metallation
 The metal reagents used in this reaction include lithium bromide and silver acetate. In this method, the metal coordinates to the nitrogen in order to activate the substrate for deprotonation. Another way to form azomethine ylides from imines is by
The metal reagents used in this reaction include lithium bromide and silver acetate. In this method, the metal coordinates to the nitrogen in order to activate the substrate for deprotonation. Another way to form azomethine ylides from imines is by prototropy
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
and by alkylation.
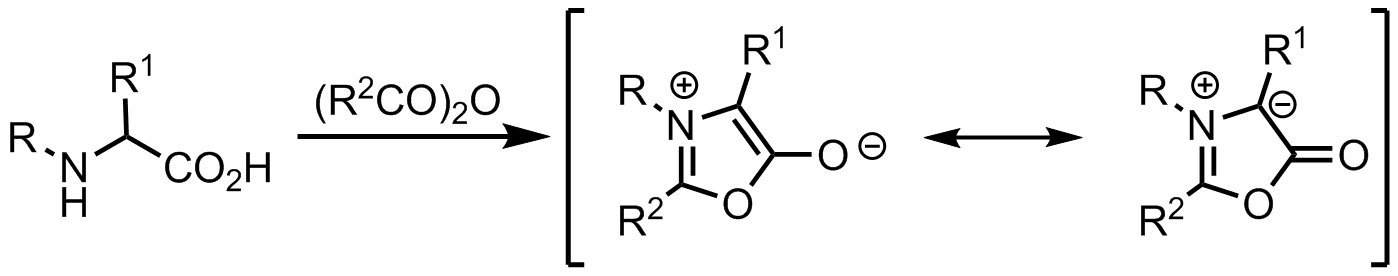
From münchnones
Ylides can be formed from münchnones, which are mesoionic heterocycles, and act as cyclic azomethine ylides.
1,3-dipolar cycloaddition reactions
 As with other cycloaddition reactions of a
As with other cycloaddition reactions of a 1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions.
The dipole has at least one resonance st ...
with a π-system, 1,3-dipolar cycloaddition using an azomethine ylide is a six-electron process. According to the Woodward–Hoffmann rules, this addition is suprafacial with respect to both the dipole and dipolarophile. The reaction is generally viewed as concerted
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not ...
, in which the two carbon-carbon bonds are being formed at the same time, but asynchronously. However, depending on the nature of the dipole and dipolarophile, diradical or zwitterionic intermediates are possible. The ''endo'' product is generally favored, as in the isoelectronic Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
. In these reactions, the azomethine ylide is typically the HOMO, and the electron-deficient dipolarophile the LUMO, although cycloaddition reactions with unactivated π-systems are known to occur, especially when the cyclization is intramolecular. For a discussion of frontier molecular orbital theory of 1,3-dipolar cycloadditions, see 1,3-dipolar cycloaddition#Frontier molecular orbital theory.
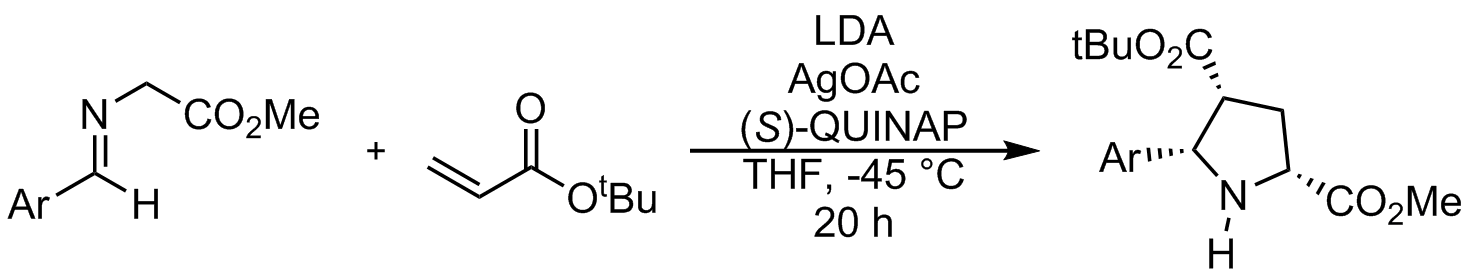 1,3-Dipolar cycloaddition reactions of azomethine ylides commonly use alkenes or
1,3-Dipolar cycloaddition reactions of azomethine ylides commonly use alkenes or alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s as dipolarophiles, to form pyrrolidines or pyrrolines Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation. 1-Pyrrolin ...
, respectively. A reaction of an azomethine ylide with an alkene is shown above, and results in a pyrrolidine. This kind of reactions can be used to synthesis Ullazine. While dipolarophiles are typically α,β-unsaturated carbonyl compounds, there have been many recent advances in developing new types of dipolarophiles.
When the dipole and dipolarophile are part of the same molecule, an intramolecular cyclization reaction can lead to a polycyclic product of considerable complexity. If the dipolarophile is tethered to a carbon of the dipole, a fused bicycle is formed. If it is tethered to the nitrogen, a bridged structure results. The intramolecular nature of the reaction can also be useful in that regioselectivity is often constrained. Another advantage to intramolecular reactions is that the dipolarophile need not be electron-deficient—many examples of cyclization reactions with electron-rich, alkyl-substituted dipolarophiles have been reported, including the synthesis of martinellic acid shown below.
Stereoselectivity of cycloadditions
Unlike most 1,3-dipolar cycloaddition reactions, in which the stereochemistry of the dipole is lost or non-existent, azomethine ylides are able to retain their stereochemistry. This is generally done by ring opening of an aziridine, and subsequent trapping by a dipolarophile before the stereochemistry can scramble. Like other 1,3-dipolar cycloaddition reactions, azomethine ylide cycloadditions can form endo or exo products. This selectivity can be tuned using metal catalysis.Enantioselective synthesis
Enantioselective cycloaddition of azomethine ylides using chiral catalysts was first described in a seminal work by Allway and Grigg in 1991. This powerful method was further developed by Jørgensen and Zhang. These reactions generally use zinc, silver, copper, nickel, and calcium complexes. Using chiral phosphine catalysts, enantiomerically pure spiroindolinones can be synthesized. The method described by Gong, et al. leads to an unexpected regiochemical outcome that does not follow electronic effects. This is attributed to favorable pi stacking with the catalyst.Other reactions
Electrocyclizations
Conjugated azomethine ylides are capable of ,5 and ,7 electrocyclizations. An example of a ,7electrocyclization of a azomethine ylide is shown below. This conrotatory ring-closing is followed by a suprafacial ,5hydride shift, which affords the rearomatized product. The sterics and geometry of the reacting phenyl ring play a major role in the success of the reaction. The compounds resulting from this type of electrocyclization have been used as dienes in
The compounds resulting from this type of electrocyclization have been used as dienes in Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
s to attach compounds to fullerenes.
Use in synthesis
Total synthesis of martinellic acid
pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
, and two stereocenters.
Total synthesis of spirotryprostatin B
In the synthesis ofspirotryprostatin B
Spirotryprostatin B is an indole, indolic alkaloid found in the ''Aspergillus fumigatus'' fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids (including Spirotryprost ...
, an azomethine ylide is formed from condensation of an amine with an aldehyde. The ylide then reacts with an electron-deficient alkene on an indolinone, resulting in formation of a spirocyclic pyrrolidine and four contiguous stereocenters.
Synthesis of benzodiazepinones
 Cyclization of an azomethine ylide with a carbonyl affords a spirocyclic oxazolidine, which loses CO2 to form a seven-membered ring. These high-utility
Cyclization of an azomethine ylide with a carbonyl affords a spirocyclic oxazolidine, which loses CO2 to form a seven-membered ring. These high-utility decarboxylative
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
multi-step reactions are common in azomethine ylide chemistry.
References
{{reflist Organic chemistry