Anodes on:
[Wikipedia]
[Google]
[Amazon]
 An anode usually is an
An anode usually is an
 The polarity of voltage on an anode with respect to an associated
The polarity of voltage on an anode with respect to an associated
 In a battery or
In a battery or 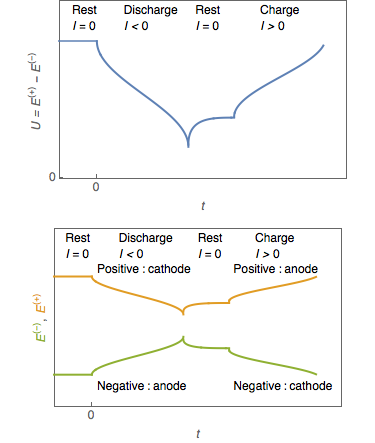 Battery manufacturers may regard the negative electrode as the anode, particularly in their technical literature. Though from an electrochemical viewpoint incorrect, it does resolve the problem of which electrode is the anode in a secondary (or rechargeable) cell. Using the traditional definition, the anode switches ends between charge and discharge cycles.
Battery manufacturers may regard the negative electrode as the anode, particularly in their technical literature. Though from an electrochemical viewpoint incorrect, it does resolve the problem of which electrode is the anode in a secondary (or rechargeable) cell. Using the traditional definition, the anode switches ends between charge and discharge cycles.
 In electronic vacuum devices such as a
In electronic vacuum devices such as a
 In a
In a
 In
In
How to define anode and cathode
{{Authority control Electrodes
electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
of a polarized electrical device
The following Outline (list), outline is provided as an overview of and topical guide to electrical polarity (also called electric polarity).
Positive and negative polarity
* In electrical engineering, electrical polarity defines the direction ...
through which conventional current enters the device. This contrasts with a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic
A mnemonic device ( ), memory trick or memory device is any learning technique that aids information retention or retrieval in the human memory, often by associating the information with something that is easier to remember.
It makes use of e ...
is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
flow, so (negatively charged) electrons flow from the anode of a galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a ...
, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode (while discharging).
In both a galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a ...
and an electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
, the anode is the electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
at which the oxidation reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
occurs. In a galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a ...
the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
, the anode is the wire or plate upon which excess positive charge is imposed. As a result of this, anions will tend to move towards the anode where they will undergo oxidation.
Historically, the anode of a galvanic cell was also known as the zincode because it was usually composed of zinc.
Charge flow
The terms anode andcathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
are not defined by the voltage polarity of electrodes, but are usually defined by the direction of current through the electrode. An anode usually is the electrode of a device through which conventional current (positive charge) flows into the device from an external circuit, while a cathode usually is the electrode through which conventional current flows out of the device.
In general, if the current through the electrodes reverses direction, as occurs for example in a rechargeable battery
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or prima ...
when it is being charged, the roles of the electrodes as anode and cathode are reversed. However, the definition of anode and cathode is different for electrical devices such as diode
A diode is a two-Terminal (electronics), terminal electronic component that conducts electric current primarily in One-way traffic, one direction (asymmetric electrical conductance, conductance). It has low (ideally zero) Electrical resistance ...
s and vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s where the electrode naming is fixed and does not depend on the actual charge flow (current). These devices usually allow substantial current flow in one direction but negligible current in the other direction. Therefore, the electrodes are named based on the direction of this "forward" current. In a diode the anode is the terminal through which current enters and the cathode is the terminal through which current leaves, when the diode is forward biased. The names of the electrodes do not change in cases where reverse current flows through the device. Similarly, in a vacuum tube only one electrode can thermionically emit electrons into the evacuated tube, so electrons can only enter the device from the external circuit through the heated electrode. Therefore, this electrode is permanently named the cathode, and the electrode through which the electrons exit the tube is named the anode.
Conventional current depends not only on the direction the charge carrier
In solid state physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. ...
s move, but also the carriers' electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
. The currents outside the device are usually carried by electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in a metal conductor. Since electrons have a negative charge, the direction of electron flow is opposite to the direction of conventional current. Consequently, electrons leave the device through the anode and enter the device through the cathode.
Examples
 The polarity of voltage on an anode with respect to an associated
The polarity of voltage on an anode with respect to an associated cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
varies depending on the device type and on its operating mode. In the following examples, the anode is negative in a device that provides power, and positive in a device that consumes power:
In a discharging battery or galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a ...
(diagram on left), the anode is the negative terminal: it is where conventional current flows into the cell. This inward current is carried externally by electrons moving outwards.
In a recharging battery, or an electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
, the anode is the positive terminal imposed by an external source of potential difference. The current through a recharging battery is opposite to the direction of current during discharge; in other words, the electrode which was the cathode during battery discharge becomes the anode while the battery is recharging.
In battery engineering, it is common to designate one electrode of a rechargeable battery the anode and the other the cathode according to the roles the electrodes play when the battery is discharged. This is despite the fact that the roles are reversed when the battery is charged. When this is done, "anode" simply designates the negative terminal of the battery and "cathode" designates the positive terminal.
In a diode
A diode is a two-Terminal (electronics), terminal electronic component that conducts electric current primarily in One-way traffic, one direction (asymmetric electrical conductance, conductance). It has low (ideally zero) Electrical resistance ...
, the anode is the terminal represented by the tail of the arrow symbol (flat side of the triangle), where conventional current flows into the device. Note the electrode naming for diodes is always based on the direction of the forward current (that of the arrow, in which the current flows "most easily"), even for types such as Zener diode
A Zener diode is a type of diode designed to exploit the Zener effect to affect electric current to flow against the normal direction from anode to cathode, when the voltage across its terminals exceeds a certain characteristic threshold, the ''Z ...
s where the current of interest is the reverse current.
In vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s or gas-filled tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Julius Plücker, Plücker tube, is an arrangement of electrodes in a gas within an dielectric, insulating, temperature-resistant envelope. Gas-filled tubes exploit phen ...
s, the anode is the terminal where current enters the tube.
Etymology
The word was coined in 1834 from theGreek
Greek may refer to:
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group
*Greek language, a branch of the Indo-European language family
**Proto-Greek language, the assumed last common ancestor of all kno ...
ἄνοδος (''anodos''), 'ascent', by William Whewell
William Whewell ( ; 24 May 17946 March 1866) was an English polymath. He was Master of Trinity College, Cambridge. In his time as a student there, he achieved distinction in both poetry and mathematics.
The breadth of Whewell's endeavours is ...
, who had been consulted by Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
over some new names needed to complete a paper on the recently discovered process of electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
. In that paper Faraday explained that when an electrolytic cell is oriented so that electric current traverses the "decomposing body" (electrolyte) in a direction "from East to West, or, which will strengthen this help to the memory, that in which the sun appears to move", the anode is where the current enters the electrolyte, on the East side: "''ano'' upwards, ''odos'' a way; the way which the sun rises".
The use of 'East' to mean the 'in' direction (actually 'in' → 'East' → 'sunrise' → 'up') may appear contrived. Previously, as related in the first reference cited above, Faraday had used the more straightforward term "eisode" (the doorway where the current enters). His motivation for changing it to something meaning 'the East electrode' (other candidates had been "eastode", "oriode" and "anatolode") was to make it immune to a possible later change in the direction convention for current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hydr ...
, whose exact nature was not known at the time. The reference he used to this effect was the Earth's magnetic field direction, which at that time was believed to be invariant. He fundamentally defined his arbitrary orientation for the cell as being that in which the internal current would run parallel to and in the same direction as a hypothetical magnetizing current loop around the local line of latitude which would induce a magnetic dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
* An electric dipole moment, electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple ...
field oriented like the Earth's. This made the internal current East to West as previously mentioned, but in the event of a later convention change it would have become West to East, so that the East electrode would not have been the 'way in' any more. Therefore, "eisode" would have become inappropriate, whereas "anode" meaning 'East electrode' would have remained correct with respect to the unchanged direction of the actual phenomenon underlying the current, then unknown but, he thought, unambiguously defined by the magnetic reference. In retrospect the name change was unfortunate, not only because the Greek roots alone do not reveal the anode's function any more, but more importantly because as we now know, the Earth's magnetic field direction on which the "anode" term is based is subject to reversals whereas the current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hydr ...
direction convention on which the "eisode" term was based has no reason to change in the future.
Since the later discovery of the electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, an easier to remember and more durably correct technically although historically false, etymology has been suggested: anode, from the Greek ''anodos'', 'way up', 'the way (up) out of the cell (or other device) for electrons'.
Electrolytic anode
Inelectrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, the ''anode'' is where oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
occurs and is the positive polarity contact in an electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
. At the anode, anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s (negative ions) are forced by the electrical potential to react chemically and give off electrons (oxidation) which then flow up and into the driving circuit. Mnemonics
A mnemonic device ( ), memory trick or memory device is any learning technique that aids information retention or retrieval in the human memory, often by associating the information with something that is easier to remember.
It makes use of e ...
: LEO Red Cat (Loss of Electrons is Oxidation, Reduction occurs at the Cathode), or AnOx Red Cat (Anode Oxidation, Reduction Cathode), or OIL RIG (Oxidation is Loss, Reduction is Gain of electrons), or Roman Catholic and Orthodox (Reduction – Cathode, anode – Oxidation), or LEO the lion says GER (Losing electrons is Oxidation, Gaining electrons is Reduction).
This process is widely used in metals refining. For example, in copper refining, copper anodes, an intermediate product from the furnaces, are electrolysed in an appropriate solution (such as sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
) to yield high purity (99.99%) cathodes. Copper cathodes produced using this method are also described as electrolytic copper.
Historically, when non-reactive anodes were desired for electrolysis, graphite (called plumbago in Faraday's time) or platinum were chosen. They were found to be some of the least reactive materials for anodes. Platinum erodes very slowly compared to other materials, and graphite crumbles and can produce carbon dioxide in aqueous solutions but otherwise does not participate in the reaction.
Battery or galvanic cell anode
galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a ...
, the anode is the negative electrode from which electrons flow out towards the external part of the circuit. Internally the positively charged cations are flowing away from the anode (even though it is negative and therefore would be expected to attract them, this is due to electrode potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can c ...
relative to the electrolyte solution being different for the anode and cathode metal/electrolyte systems); but, external to the cell in the circuit, electrons are being pushed out through the negative contact and thus through the circuit by the voltage potential as would be expected.
 Battery manufacturers may regard the negative electrode as the anode, particularly in their technical literature. Though from an electrochemical viewpoint incorrect, it does resolve the problem of which electrode is the anode in a secondary (or rechargeable) cell. Using the traditional definition, the anode switches ends between charge and discharge cycles.
Battery manufacturers may regard the negative electrode as the anode, particularly in their technical literature. Though from an electrochemical viewpoint incorrect, it does resolve the problem of which electrode is the anode in a secondary (or rechargeable) cell. Using the traditional definition, the anode switches ends between charge and discharge cycles.
Vacuum tube anode
cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
, the anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
is the positively charged electron collector. In a tube, the anode is a charged positive plate that collects the electrons emitted by the cathode through electric attraction. It also accelerates the flow of these electrons.
Diode anode
semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
diode
A diode is a two-Terminal (electronics), terminal electronic component that conducts electric current primarily in One-way traffic, one direction (asymmetric electrical conductance, conductance). It has low (ideally zero) Electrical resistance ...
, the anode is the P-doped layer which initially supplies holes
A hole is an opening in or through a particular medium, usually a solid body. Holes occur through natural and artificial processes, and may be useful for various purposes, or may represent a problem needing to be addressed in many fields of en ...
to the junction. In the junction region, the holes supplied by the anode combine with electrons supplied from the N-doped region, creating a depleted zone. As the P-doped layer supplies holes to the depleted region, negative dopant ions are left behind in the P-doped layer ('P' for positive charge-carrier ions). This creates a base negative charge on the anode. When a positive voltage is applied to anode of the diode from the circuit, more holes are able to be transferred to the depleted region, and this causes the diode to become conductive, allowing current to flow through the circuit. The terms anode and cathode should not be applied to a Zener diode
A Zener diode is a type of diode designed to exploit the Zener effect to affect electric current to flow against the normal direction from anode to cathode, when the voltage across its terminals exceeds a certain characteristic threshold, the ''Z ...
, since it allows flow in either direction, depending on the polarity of the applied potential (i.e. voltage).
Sacrificial anode
 In
In cathodic protection
Cathodic protection (CP; ) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded " sacrifi ...
, a metal anode that is more reactive to the corrosive environment than the metal system to be protected is electrically linked to the protected system. As a result, the metal anode partially corrodes or dissolves instead of the metal system. As an example, an iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
or steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
ship's hull may be protected by a zinc sacrificial anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion.
They are made from a metal alloy with a more "active" voltage (more n ...
, which will dissolve into the seawater and prevent the hull from being corroded. Sacrificial anodes are particularly needed for systems where a static charge
Static electricity is an imbalance of electric charges within or on the surface of a material. The charge remains until it can move away by an electric current or electrical discharge. The word "static" is used to differentiate it from curren ...
is generated by the action of flowing liquids, such as pipelines and watercraft. Sacrificial anodes are also generally used in tank-type water heaters.
In 1824 to reduce the impact of this destructive electrolytic action on ships hulls, their fastenings and underwater equipment, the scientist-engineer Humphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
developed the first and still most widely used marine electrolysis protection system. Davy installed sacrificial anodes made from a more electrically reactive (less noble) metal attached to the vessel hull and electrically connected to form a cathodic protection circuit.
A less obvious example of this type of protection is the process of galvanising
Galvanization ( also spelled galvanisation) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are coated by submerging them in a bath of ...
iron. This process coats iron structures (such as fencing) with a coating of zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
metal. As long as the zinc remains intact, the iron is protected from the effects of corrosion. Inevitably, the zinc coating becomes breached, either by cracking or physical damage. Once this occurs, corrosive elements act as an electrolyte and the zinc/iron combination as electrodes. The resultant current ensures that the zinc coating is sacrificed but that the base iron does not corrode. Such a coating can protect an iron structure for a few decades, but once the protecting coating is consumed, the iron rapidly corrodes.
If, conversely, tin is used to coat steel, when a breach of the coating occurs it actually accelerates oxidation of the iron.
Impressed current anode
Another cathodic protection is used on the impressed current anode. It is made from titanium and covered with mixed metal oxide. Unlike the sacrificial anode rod, the impressed current anode does not sacrifice its structure. This technology uses an external current provided by a DC source to create the cathodic protection. Impressed current anodes are used in larger structures like pipelines, boats, city water tower, water heaters and more.Related antonym
The opposite of an anode is acathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
. When the current through the device is reversed, the electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s switch functions, so the anode becomes the cathode and the cathode becomes anode, as long as the reversed current is applied. The exception is diodes where electrode naming is always based on the forward current direction.
See also
*Anodizing
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
The process is called ''anodizing'' because the part to be treated forms the anode electrode of an electr ...
* Galvanic anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion.
They are made from a metal alloy with a more "active" voltage (more n ...
* Gas-filled tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Julius Plücker, Plücker tube, is an arrangement of electrodes in a gas within an dielectric, insulating, temperature-resistant envelope. Gas-filled tubes exploit phen ...
* Primary cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and it is not rechargeable unlike a secondary cell ( rechargeable battery). In general, the electrochemical reaction occurring in ...
* Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
(reduction–oxidation)
References
External links
How to define anode and cathode
{{Authority control Electrodes