|
Galvanic Cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane. Volta was the inventor of the voltaic pile, the first electrical battery. Common usage of the word ''battery'' has evolved to include a single Galvanic cell, but the first batteries had many Galvanic cells. History In 1780, Luigi Galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same time to two different parts of a muscle of a frog leg, to close the circuit, the frog's leg contracts. He called this " animal electricity". The frog's leg, as well as being a detector ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Cell With No Cation Flow
Galvanic (after Luigi Galvani) may refer to: * Galvanic anode * Galvanic bath * Galvanic cell * Galvanic corrosion Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, different metal, when both in the prese ... * Galvanic current * Galvanic isolation * Galvanic potential * Galvanic series * Galvanic skin response * Galvanic vestibular stimulation * Galvanism * Galvanization * Operation Galvanic, World War II attack which included the Battle of Tarawa See also * List of forms of electricity named after scientists {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daniell Cell
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
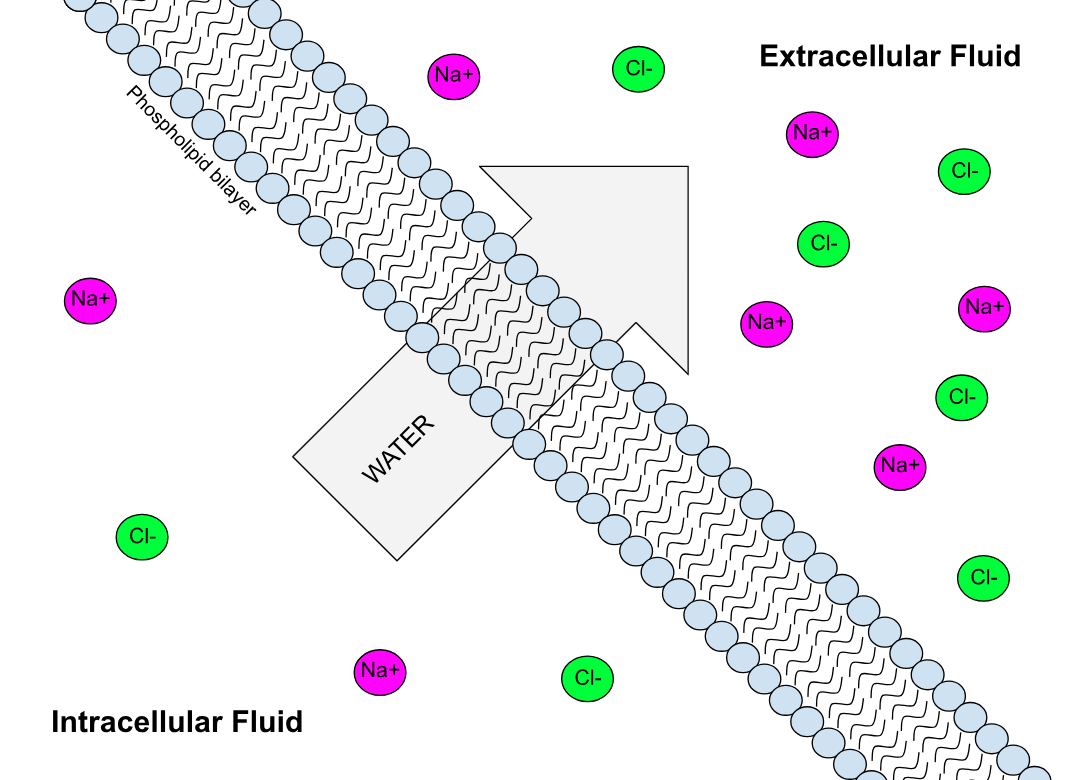
Semi-permeable Membrane
Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes Phospholipid bilayer A phospholipid bilayer is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-cell
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical anode reaction involves a metal atom in the electrode being dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions. This self-limiting action occurs almost instantly in an isolated half-cell; in applications two dissimilar half-cells are appropriately connected to constitute a Galvanic cell. A standa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work (physics), work W that was done against constant external pressure P_\text to establish the system's physical dimensions from V_\text=0 to some final volume V_\text (as W=P_\text\Delta V), i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; Bond energy, bond, Lattice energy, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Cell Labeled
Galvanic (after Luigi Galvani) may refer to: * Galvanic anode * Galvanic bath * Galvanic cell * Galvanic corrosion * Galvanic current * Galvanic isolation * Galvanic potential * Galvanic series * Galvanic skin response Electrodermal activity (EDA) is the property of the human body that causes continuous variation in the electrical characteristics of the skin. Historically, EDA has also been known as skin conductance, galvanic skin response (GSR), electroderm ... * Galvanic vestibular stimulation * Galvanism * Galvanization * Operation Galvanic, World War II attack which included the Battle of Tarawa See also * List of forms of electricity named after scientists {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parthia
Parthia ( ''Parθava''; ''Parθaw''; ''Pahlaw'') is a historical region located in northeastern Greater Iran. It was conquered and subjugated by the empire of the Medes during the 7th century BC, was incorporated into the subsequent Achaemenid Empire under Cyrus the Great in the 6th century BC, and formed part of the Hellenistic Seleucid Empire after the Wars of Alexander the Great, 4th-century BC conquests of Alexander the Great. The region later served as the political and cultural base of the Eastern Iranian languages, Eastern Iranian Parni people and Arsacid dynasty, rulers of the Parthian Empire (247 BC – 224 AD). The Sasanian Empire, the last state of History of Iran, pre-Islamic Iran, also held the region and maintained the Seven Great Houses of Iran, seven Parthian clans as part of their feudal aristocracy. Name The name "Parthia" is a continuation from Latin language, Latin ', from Old Persian ', which was the Parthian language self-designator signifying "of the Pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baghdad Battery
The Baghdad Battery is the name given to a set of three artifacts which were found together: a ceramic pot, a tube of copper, and a rod of iron. It was discovered in present-day Khujut Rabu, Iraq in 1936, close to the ancient city of Ctesiphon, the capital of the Parthian (150 BC – 223 AD) and Sasanian (224–650 AD) empires, and it is believed to date from either of these periods. Its origin and purpose remain unclear. Wilhelm König, at the time director of the laboratory of the National Museum of Iraq, suggested that the object functioned as a galvanic cell, possibly used for electroplating, or some kind of electrotherapy. There is no electroplated object known from this period, and the claims are universally rejected by archaeologists. An alternative explanation is that it functioned as a storage vessel for sacred scrolls. Ten similar clay vessels had been found earlier. Four were found in 1930 in Seleucia dating to the Sassanid period. Three were sealed with bitumen and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm König
Wilhelm König (born in Vienna) was an Austrian archaeologist and painter. A painter by profession, in 1931, König was elected assistant to the German leader of the Baghdad Antiquity Administration with the title of a "Direktor". At the excavation of a Parthian settlement in modern day Khujut Rabu (near Baghdad, Iraq), he discovered the alleged Baghdad Battery. In February 1939, he returned to Vienna, due to blood poisoning, where he published a book ''Im verlorenen Paradies. Neun Jahre Irak''. Controversy In March 2012, Professor Elizabeth Stone, of Stony Brook University, an expert on Iraqi archaeology, returning from one of the first archaeological expeditions in Iraq since 20 years, stated that she does not know a single archaeologist, who believed that this was a "''real'' battery".Prof. Stone's statement, listed as a 'red flag' amon5 red flags why it was not a battery(with sources, on Archaeology Fantasies website) Works''Neun Jahre Irak''Brünn, Münster, Wien 1940 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromotive Force
In electromagnetism and electronics, electromotive force (also electromotance, abbreviated emf, denoted \mathcal) is an energy transfer to an electric circuit per unit of electric charge, measured in volts. Devices called electrical ''transducers'' provide an emf by Energy transformation, converting other forms of energy into electrical energy. Other types of electrical equipment also produce an emf, such as Battery (electricity), batteries, which convert chemical energy, and Electric generator, generators, which convert mechanical energy. This energy conversion is achieved by Force, physical forces applying Work (physics), physical work on electric charges. However, electromotive force itself is not a physical force, and ISO/International Electrotechnical Commission, IEC standards have deprecated the term in favor of source voltage or source tension instead (denoted U_s). An Hydraulic analogy, electronic–hydraulic analogy may view emf as the mechanical work done to water by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |