Anammox Mechanisms on:
[Wikipedia]
[Google]
[Amazon]
Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the
patent WO9807664
/ref> by the
 In this biological process, which is a
In this biological process, which is a
 In 1932, it was reported that
In 1932, it was reported that
 According to labeling experiments carried out in 1997,
According to labeling experiments carried out in 1997,
The first step is the partial
/ref> This makes it difficult to grow enough sludge for a wastewater treatment reactor. Also the recovery time after the loss of sludge by accident is longer than in conventional nitrogen removal systems. On the other hand, this slow growing rate is an advantage due to the reduction of surplus sludge that needs to be removed and treated. Depending on the exact species, the optimum pH level is 8. Therefore, it can be necessary to adjust the pH of the wastewater by adding caustic.
nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
and ammonium ion
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula or . It is formed by the addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium ...
s are converted directly into diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and water.
The bacteria that perform the anammox process are genera that belong to the bacterial phylum Planctomycetota
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammoni ...
. The anammox bacteria all possess one anammoxosome, a lipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes form a continuous barrier around all cell (biology), cells. The cell membranes of almost all organisms and many viruses a ...
membrane-bound compartment inside the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
in which the anammox process takes place. The anammoxosome membranes are rich in ladderane
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the ...
lipids; the presence of these lipids is so far unique in biology.
"Anammox" is also the trademarked name for an anammox-based ammonium removal technology developedJetten Michael Silvester Maria, Van Loosdrecht Marinus Corneli; Technische Universiteit Delftpatent WO9807664
/ref> by the
Delft University of Technology
The Delft University of Technology (TU Delft; ) is the oldest and largest Dutch public university, public Institute of technology, technical university, located in Delft, Netherlands. It specializes in engineering, technology, computing, design, a ...
.
Process background
 In this biological process, which is a
In this biological process, which is a redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
comproportionation
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionatio ...
reaction, nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
and ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
ions are converted directly into a diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear mol ...
of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and water.
: (Δ''G''° = ).
Globally, this process may be responsible for 30–50% of the gas produced in the oceans. It is thus a major sink for fixed nitrogen
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiologically in chemical industries. Biological nitrogen fixation or ''diazotrophy'' is catalyzed by enz ...
and so limits oceanic primary productivity.
The bacteria that perform the anammox process belong to the bacterial phylum Planctomycetota
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammoni ...
. Currently, five anammox genera have been discovered: '' Brocadia'', ''Kuenenia'', ''Anammoxoglobus'', ''Jettenia'' (all fresh water species), and '' Scalindua'' (marine species). The anammox bacteria are characterized by several striking properties:
* They all possess one anammoxosome, a membrane bound compartment inside the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
which is the locus of anammox catabolism. Further, the membranes of these bacteria mainly consist of ladderane
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the ...
lipids so far unique in biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
.
* Of special interest is the conversion to hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
(normally used as a high-energy rocket fuel, and poisonous to most living organisms) as an intermediate.
* A final striking feature of the organism is the extremely slow growth rate; the doubling time
The doubling time is the time it takes for a population to double in size/value. It is applied to population growth, inflation, resource extraction, consumption of goods, compound interest, the volume of malignant tumours, and many other things t ...
is anywhere from 7–22 days.
The anammox bacteria are geared towards converting their substrates at very low concentrations; in other words, they have a very high affinity to their substrates ammonium and nitrite (sub-micromolar range). Anammox cells are packed with cytochrome c type proteins (≈30% of the protein complement), including the enzymes that perform the key catabolic reactions of the anammox process, making the cells remarkably red. The anammox process was originally found to occur only from 20 °C to 43 °C
but more recently, anammox has been observed at temperatures from 36 °C to 52 °C in hot springs and 60 °C to 85 °C at hydrothermal vents located along the Mid-Atlantic Ridge.
History
 In 1932, it was reported that
In 1932, it was reported that dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
gas was generated via an unknown mechanism during fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
in the sediments of Lake Mendota, Wisconsin, USA. In 1965, F. A. Richards noticed that most of the ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
that should be produced during the anaerobic remineralization of organic matter was unaccounted for. As there was no known biological pathway for this transformation, biological anaerobic oxidation of ammonium received little further attention.
In 1977, Engelbert Broda predicted the existence of two chemolithoautotrophic microorganisms capable of oxidizing ammonium to dinitrogen gas on the basis of thermodynamic calculations. It was thought that anaerobic oxidation of ammonium would not be feasible, assuming that the predecessors had tried and failed to establish a biological basis for those reactions. By the 1990s, Arnold Mulder's observations were just consistent with Richard's suggestion. In their anoxic denitrifying pilot reactor, ammonium disappeared at the expense of nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
with a clear nitrogen production. The reactor used the effluent from a methanogenic pilot reactor, which contained ammonium, sulphide and other compounds, and nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
from a nitrifying plant as the influent. The process was named "anammox," and was realized to have great significance in the removal of unwanted ammonium.
The discovery of the anammox process was first publicly presented at the 5th European congress on biotechnology
Biotechnology is a multidisciplinary field that involves the integration of natural sciences and Engineering Science, engineering sciences in order to achieve the application of organisms and parts thereof for products and services. Specialists ...
. By the mid-1990s, the discovery of anammox in the fluidized bed reactor was published. A maximum ammonium removal rate of 0.4 kg N/m3/d was achieved. It was shown that for every mole of ammonium consumed, 0.6 mol of nitrate was required, resulting in the formation of 0.8 mol of gas.
In 1995, the biological nature of anammox was identified. Labeling experiments with in combination with showed that 14-15 was the dominant product making up 98.2% of the total labeled . It was realized that, instead of nitrate, nitrite was assumed as the oxidizing agent of ammonium in anammox reaction. Based on a previous study, Strous et al. calculated the stoichiometry of anammox process by mass balancing, which is widely accepted by other groups. Later, anammox bacteria were identified as Planctomycetota
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammoni ...
, and the first identified anammox organism was named ''Candidatus
In prokaryote nomenclature, ''Candidatus'' (abbreviated ''Ca.''; Latin for "candidate of Roman office") is used to name prokaryotic taxa that are well characterized but yet- uncultured. Contemporary sequencing approaches, such as 16S ribosomal R ...
'' " Brocadia anammoxidans."
Before 2002, anammox was assumed to be a minor player in the nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
within natural ecosystems. In 2002 however, anammox was found to play an important part in the biological nitrogen cycle, accounting for 24–67% of the total production in the continental shelf sediments that were studied. The discovery of anammox process modified the concept of biological nitrogen cycle, as depicted in Figure 2.
Possible reaction mechanisms

 According to labeling experiments carried out in 1997,
According to labeling experiments carried out in 1997, ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
is biologically oxidized by hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Prof ...
, most likely derived from nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
, as the probable electron acceptor. The conversion of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
to dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
gas is hypothesized to be the reaction that generates the electron equivalents for the reduction of nitrite to hydroxylamine. In general, two possible reaction mechanisms are addressed:
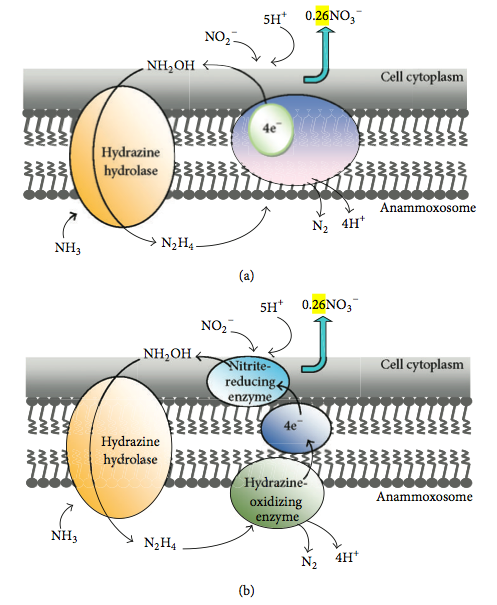
* One mechanism hypothesizes that a membrane-bound enzyme complex converts ammonium and hydroxylamine to hydrazine first, followed by the oxidation of hydrazine to dinitrogen gas in the periplasm. At the same time, nitrite is reduced to hydroxylamine at the cytoplasmic site of the same enzyme complex responsible for hydrazine oxidation with an internal electron transport (Figure 3a).
* The other mechanism postulates the following: ammonium and hydroxylamine are converted to hydrazine by a membrane-bound enzyme complex, hydrazine is oxidized in the periplasm to dinitrogen gas, and the generated electrons are transferred via an electron transport chain to nitrite reducing enzyme in the cytoplasm where nitrite is reduced to hydroxylamine (Figure 3b).
Whether the reduction of nitrite and the oxidation of hydrazine occur at different sites of the same enzyme or the reactions are catalyzed by different enzyme systems connected via an electron transport chain remains to be investigated. In microbial nitrogen metabolism, the occurrence of hydrazine as an intermediate is rare. Hydrazine has been proposed as an enzyme-bound intermediate in the nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
reaction.
Recently, using detailed molecular analyses and combining complementary methods, Kartal and coworkers published strong evidence supporting the latter mechanism.
Furthermore, the enzyme producing hydrazine, hydrazine synthase was purified and shown to produce hydrazine from NO and ammonium. The production of hydrazine from ammonium and NO was also supported by the resolution of the crystal structure of the enzyme hydrazine synthase.
A possible role of nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
(NO) or nitroxyl
Nitroxyl (common name) or azanone (IUPAC name) is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in solution. Its conjugate base, NO−, the nitroxide anion, is the reduced form o ...
(HNO) in anammox was proposed by Hooper et al. by way of condensation of NO or HNO and ammonium on an enzyme related to the ammonium monooxygenase family. The formed hydrazine or imine could subsequently be converted by the enzyme hydroxylamine oxidase to dinitrogen gas, and the reducing equivalents produced in the reaction are required to combine NO or HNO and ammonium or to reduce nitrite to NO. Environmental genomics analysis of the species ''Candidatus'' '' Kuenenia stuttgartiensis'', through a slightly different and complementary metabolism mechanism, suggested NO to be the intermediate instead of hydroxylamine (Figure 4). However, this hypothesis also agreed that hydrazine was an important intermediate in the process. In this pathway (Figure 4), there are two enzymes unique to anammox bacteria: hydrazine synthase (hzs) and hydrazine dehydrogenase (hdh). The HZS produces hydrazine from nitric oxide and ammonium, and HDH transfer the electrons from hydrazine to ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
. Few new genes, such as some known fatty acid biosynthesis and S-adenosylmethionine radical enzyme genes, containing domains involved in electron transfer and catalysis have been detected. Anammox microorganisms can also directly couple NO reduction to ammonia oxidation, without the need for nitrite supply.
Another, still unexplored, reaction mechanism involves anaerobic ammonium oxidation on anodes
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devi ...
of bio-electrical systems. Such systems can be microbial fuel cells or microbial electrolysis cells. In the absence of dissolved oxygen, nitrite, or nitrate, microbes living in the anode compartment are able to oxidize ammonium to dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh ...
gas (N2) just as in the classical anammox process. At the same time, they unload the liberated electrons onto the anode, producing electrical current. This electrical current can be used either directly in fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
mode or for hydrogen and methane gas production in electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
mode. While there is no clarity on the reaction mechanism behind, one hypothesis is that nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
, or dinitrogen oxide play a role as intermediates. However, since the process occurs at very low electrochemical potentials
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typica ...
, other, more speculative, reaction mechanisms seem possible as well.
The Anammoxosome
The anammoxosome is a membrane-bound cellular compartment responsible for containing anammox processes and their associated enzymes. This compartment occupies the majority of thecytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
of anammox bacteria and is composed of a single lipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes form a continuous barrier around all cell (biology), cells. The cell membranes of almost all organisms and many viruses a ...
enriched in a unique combination of ester-linked (mainly found in bacteria and eukarya), ether-linked (mostly found in archaea), and ladderane lipids (unique to anammox bacteria). These lipids modulate the fluidity of the anammoxosome membrane, with the ladderane lipids providing the membrane rigidity, resulting in decreased membrane permeability to hydroxide and hydrogen ions. In addition to these ions, it is thought that increased rigidity prevents all byproducts and intermediates – such as hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
, that may be toxic to the cell – from permeating from the compartment, protecting the rest of the cell from damage. When anammox bacteria divide, the anammoxosome is also divided evenly between daughter cells.
The anammoxosome is analogous to eukaryotic mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
both structurally and functionally. Structurally speaking, the anammoxosome membrane is highly folded, similar to the cristae found in mitochondria. This feature serves to increase the surface area of the membrane to increase the amount of proteins that can be embedded within it to maximize its metabolic efficiency. Another function of anammoxosome folding is to create binding sites for proteins that are specific to the topology of the folded membrane. In addition to folds, tubule-like structures have been found protruding into the lumen of the anammoxosome; however, the function of these structures remains unclear. The function of the anammoxosome in metabolism is similar to the mitochondria as both serve as the site of catabolic reactions, an electron transport chain, and ATP synthesis. The compartment is home to the enzymes that perform anammox processes such as a variant of hydroxylamine oxidoreductase and hydrazine synthase. Oxidation of the different molecules along the process provides electrons to an electron transport chain mediated by cytochrome C proteins and different heme molecules. This chain pumps hydrogen ions from the cytoplasm of the cell into the lumen to establish a membrane potential and ultimately pass through an F1FO ATP synthase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed ...
to synthesize ATP.
One additional feature found within the anammoxosome is the presence of iron storage proteins. While the purpose of these inclusions is unclear, one explanation for their presence suggests that they may have a role in response to an iron-limiting environment. Iron that is stored in these proteins may be used in limiting environments for iron respiration or for use in heme proteins involved in the electron transport chain of the anammoxosome.
Species diversity
Until now, ten anammox species have been described, including seven that are available in laboratory enrichment cultures. All have the taxonomical status of ''Candidatus
In prokaryote nomenclature, ''Candidatus'' (abbreviated ''Ca.''; Latin for "candidate of Roman office") is used to name prokaryotic taxa that are well characterized but yet- uncultured. Contemporary sequencing approaches, such as 16S ribosomal R ...
'', as none were obtained as classical pure cultures. Known species are divided over five genera:
# '' Kuenenia'', one species: '' Kuenenia stuttgartiensis''.
# '' Brocadia'', three species: '' B. anammoxidans'', '' B. fulgida'', and '' B. sinica''.
# '' Anammoxoglobus'', one species: '' A. propionicus''.
# '' Jettenia'', one species: '' J. asiatica''.
# '' Scalindua'', four species: '' S. brodae'', '' S. sorokinii'', '' S. wagneri'', and '' S. profunda.''
Representatives of the first four genera were enriched from sludge from wastewater treatment plants; ''K. stuttgartiensis'', ''B. anammoxidans'', ''B. fulgida'', and ''A. propionicus'' were even obtained from the same inoculum. ''Scalindua'' dominates the marine environment, but is also found in some freshwater ecosystems and wastewater treatment plants.
Together, these 10 species likely only represent a minute fraction of anammox biodiversity. For instance, there are currently over 2000 16S rRNA gene sequences affiliated with anammox bacteria that have been deposited to the Genbank (https://www.ncbi.nlm.nih.gov/genbank/), representing an overlooked continuum of species, subspecies, and strains, each apparently having found its specific niche in the wide variety of habitats where anammox bacteria are encountered. Species microdiversity is particularly impressive for the marine representative ''Scalindua''. A question that remains to be investigated is which environmental factors determine species differentiation among anammox bacteria.
The sequence identities of the anammox 16S rRNA genes range from 87 to 99%, and phylogenetic analysis places them all within the phylum ''Planctomycetota'', which form the PVC superphylum together with ''Verrucomicrobia'' and ''Chlamydiae''. Within the ''Planctomycetota'', anammox bacteria deeply branch as a monophyletic clade. Their phylogenetic position together with a broad range of specific physiological, cellular, and molecular traits give anammox bacteria their own order ''Brocadiales''.
Application in wastewater treatment
The application of the anammox process lies in the removal ofammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
in the wastewater treatment and consists of two separate processes.The first step is the partial
nitrification
''Nitrification'' is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or ent ...
(nitritation) of half of the ammonium to nitrite by ammonia oxidizing bacteria:
: 2 + 3 → 2 + 4 + 2
The remaining half of the ammonium and the newly formed nitrite are converted in the anammox process to diatomic nitrogen gas and nitrate (not shown) by anammox bacteria:
: + → + 2
Both processes can take place in 1 reactor where two guilds of bacteria form compact granules.
For the enrichment of the anammox organisms a granular biomass or biofilm
A biofilm is a Syntrophy, syntrophic Microbial consortium, community of microorganisms in which cell (biology), cells cell adhesion, stick to each other and often also to a surface. These adherent cells become embedded within a slimy ext ...
system seems to be especially suited in which the necessary sludge age of more than 20 days can be ensured. Possible reactors are sequencing batch reactor
Sequencing batch reactors (SBR) or sequential batch reactors are a type of activated sludge process for the wastewater treatment, treatment of wastewater. SBRs treat wastewater such as sewage or output from anaerobic digesters or mechanical biolog ...
s (SBR), moving bed reactors or gas-lift-loop reactors. The cost reduction compared to conventional nitrogen removal is considerable; the technique is still young but proven in several fullscale installations.
The first full scale reactor intended for the application of anammox bacteria was built in the Netherlands in 2002. In other wastewater treatment plants, such as the one in Germany (Hattingen), anammox activity is coincidentally observed though were not built for that purpose. As of 2006, there are three full scale processes in The Netherlands: one in a municipal wastewater treatment plant (in Rotterdam
Rotterdam ( , ; ; ) is the second-largest List of cities in the Netherlands by province, city in the Netherlands after the national capital of Amsterdam. It is in the Provinces of the Netherlands, province of South Holland, part of the North S ...
), and two on industrial effluent. One is a tannery
Tanning, or hide tanning, is the process of treating skins and hides of animals to produce leather. A tannery is the place where the skins are processed.
Historically, vegetable based tanning used tannin, an acidic chemical compound derived fr ...
, the other a potato processing plant.
Advantages
Conventional nitrogen removal from ammonium-rich wastewater is accomplished in two separate steps: nitrification, which is mediated by aerobic ammonia- and nitrite-oxidizing bacteria and denitrification carried out by denitrifiers, which reduce nitrate to with the input of suitable electron donors. Aeration and input of organic substrates (typically methanol) show that these two processes are: # Highly energy consuming. # Associated with the production of excess sludge. # Produce significant amounts of green-house gases such as and and ozone-depleting NO. Because anammox bacteria convert ammonium and nitrite directly to anaerobically, this process does not require aeration and other electron donors. Nevertheless, oxygen is still required for the production of nitrite by ammonia-oxidizing bacteria. However, in partial nitritation/anammox systems, oxygen demand is greatly reduced because only half of the ammonium needs to be oxidized to nitrite instead of full conversion to nitrate. The autotrophic nature of anammox bacteria and ammonia-oxidizing bacteria guarantee a low yield and thus less sludge production. Additionally, anammox bacteria easily form stable self-aggregated biofilm (granules) allowing reliable operation of compact systems characterized by high biomass concentration and conversion rate up to 5–10 kg N m−3. Overall, it has been shown that efficient application of the anammox process in wastewater treatment results in a cost reduction of up to 60% as well as lower emissions.Disadvantages
The doubling time is slow, between 10 days to 2 weeks.microbewiki: Anammox/ref> This makes it difficult to grow enough sludge for a wastewater treatment reactor. Also the recovery time after the loss of sludge by accident is longer than in conventional nitrogen removal systems. On the other hand, this slow growing rate is an advantage due to the reduction of surplus sludge that needs to be removed and treated. Depending on the exact species, the optimum pH level is 8. Therefore, it can be necessary to adjust the pH of the wastewater by adding caustic.
References
{{Reflist Biochemical reactions Environmental microbiology Nitrogen cycle