alkylating agents on:
[Wikipedia]
[Google]
[Amazon]
 Alkylation is a
Alkylation is a
 The SN2 mechanism is not available for aryl substituents, where the trajectory to attack the carbon atom would be inside the ring. Thus, only reactions catalyzed by organometallic catalysts are possible.
The SN2 mechanism is not available for aryl substituents, where the trajectory to attack the carbon atom would be inside the ring. Thus, only reactions catalyzed by organometallic catalysts are possible.
R-OH + R'-X -> R-O-R'
When the alkylating agent is an alkyl halide, the conversion is called the Williamson ether synthesis.
Alcohols are also good alkylating agents in the presence of suitable acid catalysts. For example, most methyl amines are prepared by alkylation of ammonia with methanol. The alkylation of phenols is particularly straightforward since it is subject to fewer competing reactions.
:Ph-O- + Me2-SO4 -> Ph-O-Me + Me-SO4-
:(with as a spectator ion)
More complex alkylation of a alcohols and phenols involve
 Electrophilic alkylating agents deliver the equivalent of an alkyl
Electrophilic alkylating agents deliver the equivalent of an alkyl
 Electrophilic alkylation uses
Electrophilic alkylation uses C2H4 + CH3CO2H -> CH3CO2C2H5

Macrogalleria page on polycarbonate production
* {{Authority control Industrial processes Oil refining Organic reactions Chemical processes
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
that entails transfer of an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
group. The alkyl group may be transferred as an alkyl carbocation, a free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of P ...
. For upgrading of petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
is used in chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy re ...
to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
Nucleophilic alkylating agents
Nucleophilic alkylating agents deliver the equivalent of analkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
( carbanion). The formal "alkyl anion" attacks an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
, forming a new covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
between the alkyl group and the electrophile. The counterion, which is a cation such as lithium, can be removed and washed away in the work-up. Examples include the use of organometallic compounds such as Grignard (organomagnesium), organolithium, organocopper, and organosodium reagents. These compounds typically can add to an electron-deficient carbon atom such as at a carbonyl group. Nucleophilic alkylating agents can displace halide substituents on a carbon atom through the SN2 mechanism. With a catalyst, they also alkylate alkyl and aryl halides, as exemplified by Suzuki couplings.
 The SN2 mechanism is not available for aryl substituents, where the trajectory to attack the carbon atom would be inside the ring. Thus, only reactions catalyzed by organometallic catalysts are possible.
The SN2 mechanism is not available for aryl substituents, where the trajectory to attack the carbon atom would be inside the ring. Thus, only reactions catalyzed by organometallic catalysts are possible.
Alkylation by carbon electrophiles
C-alkylation
C-alkylation is a process for the formation of carbon-carbon bonds. The largest example of this takes place in the alkylation units of petrochemical plants, which convert low-molecular-weightalkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
into high octane gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
components. Electron-rich species such as phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s are also commonly alkylated to produce a variety of products; examples include linear alkylbenzenes used in the production of surfactants like LAS, or butylated phenols like BHT, which are used as antioxidant
Antioxidants are Chemical compound, compounds that inhibit Redox, oxidation, a chemical reaction that can produce Radical (chemistry), free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants ...
s. This can be achieved using either acid catalysts like Amberlyst, or Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s like aluminium. On a laboratory scale the Friedel–Crafts reaction uses alkyl halides, as these are often easier to handle than their corresponding alkenes, which tend to be gasses. The reaction is catalysed by aluminium trichloride. This approach is rarely used industrially as alkyl halides are more expensive than alkenes.
N-,P-, S- alkylation
N-, P-, and S-alkylation are important processes for the formation of carbon-nitrogen, carbon-phosphorus, and carbon-sulfur bonds, Amines are readily alkylated. The rate of alkylation follows the order tertiary amine < secondary amine < primary amine. Typical alkylating agents are alkyl halides. Industry often relies ongreen chemistry
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. Wh ...
methods involving alkylation of amines with alcohols, the byproduct being water. Hydroamination is another green method for N-alkylation.
In the Menshutkin reaction, a tertiary amine is converted into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides, the products being phosphonium salts.
Thiols are readily alkylated to give thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s via the thiol-ene reaction. The reaction is typically conducted in the presence of a base or using the conjugate base of the thiol. Thioethers undergo alkylation to give sulfonium ions.
O-alkylation
Alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s alkylate to give ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s:
:ethoxylation
In organic chemistry, ethoxylation is a chemical reaction in which ethylene oxide () adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.
In the usual application, alcoh ...
. Ethylene oxide is the alkylating group in this reaction.
Oxidative addition to metals
In the process called oxidative addition, low-valent metals often react with alkylating agents to give metal alkyls. This reaction is one step in the Cativa process for the synthesis ofacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
from methyl iodide. Many cross coupling reactions proceed via oxidative addition as well.
Electrophilic alkylating agents
 Electrophilic alkylating agents deliver the equivalent of an alkyl
Electrophilic alkylating agents deliver the equivalent of an alkyl cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. Alkyl halides are typical alkylating agents. Trimethyloxonium tetrafluoroborate and triethyloxonium tetrafluoroborate are particularly strong electrophiles due to their overt positive charge and an inert leaving group (dimethyl or diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
). Dimethyl sulfate
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as ( CH3)2 SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agen ...
is intermediate in electrophilicity.
Methylation with diazomethane
Diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
is a popular methylating agent in the laboratory, but it is too hazardous (explosive gas with a high acute toxicity) to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
Hazards
Electrophilic, soluble alkylating agents are often toxic and carcinogenic, due to their tendency to alkylate DNA. This mechanism of toxicity is relevant to the function of anti-cancer drugs in the form ofalkylating antineoplastic agents
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA.
Since cancer cells, in general, proliferate faster and with less error-correcting than healthy cells, cancer cells a ...
. Some chemical weapons such as mustard gas (sulfide of dichloroethyl) function as alkylating agents. Alkylated DNA either does not coil or uncoil properly, or cannot be processed by information-decoding enzymes. Without functional DNA, the functioning of the cell ceases, leading to cell death. Thus, these alkylating agents are cytotoxic.
Catalysts
Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s and Brønsted acids, sometimes both. Classically, Lewis acids, e.g., aluminium trichloride, are employed when the alkyl halide are used. Brønsted acids are used when alkylating with olefins. Typical catalysts are zeolites, i.e. solid acid catalysts, and sulfuric acid. Silicotungstic acid is used to manufacture ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
by the alkylation of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
by ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
:
:In biology
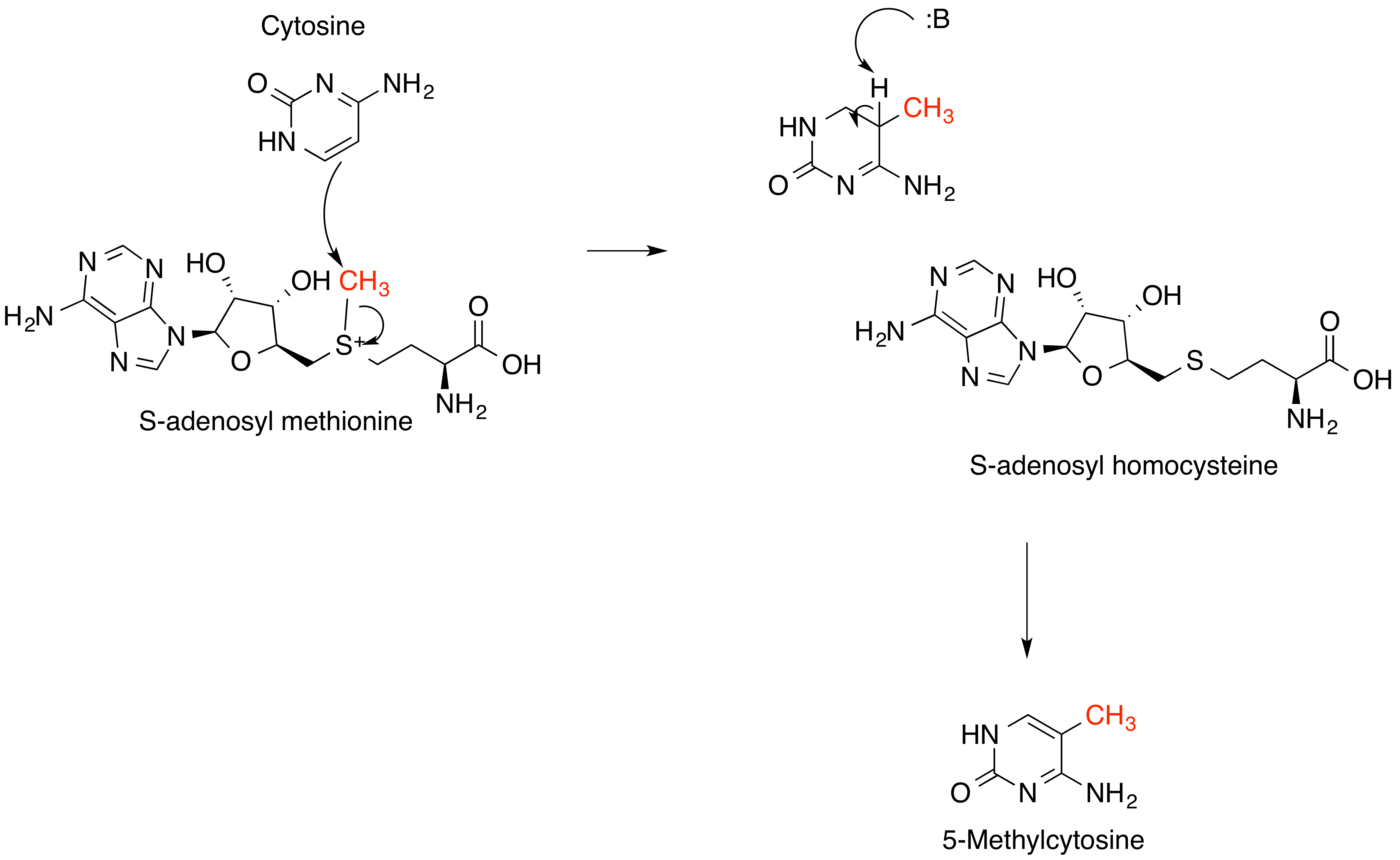
Alkylation in biology causes DNA damage. It is the transfer of alkyl groups to the nitrogenous bases. It is caused by alkylating agents such as EMS (Ethyl Methyl Sulphonate). Bifunctional alkyl groups which have two alkyl groups in them cause cross linking in DNA. Alkylation damaged ring nitrogen bases are repaired via the Base Excision Repair (BER) pathway.
Commodity chemicals
Several commodity chemicals are produced by alkylation. Included are several fundamental benzene-based feedstocks such as ethylbenzene (precursor to styrene), cumene (precursor tophenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
and acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
), linear alkylbenzene sulfonates (for detergents).
Gasoline production
In a conventionaloil refinery
An oil refinery or petroleum refinery is an industrial processes, industrial process Factory, plant where petroleum (crude oil) is transformed and refining, refined into products such as gasoline (petrol), diesel fuel, Bitumen, asphalt base, ...
, isobutane is alkylated with low-molecular-weight alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s (primarily a mixture of propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like od ...
and butene) in the presence of a Brønsted acid catalyst, which can include solid acids (zeolites). The catalyst protonates the alkenes (propene, butene) to produce carbocations, which alkylate isobutane. The product, called "alkylate", is composed of a mixture of high-octane
Octane is a hydrocarbon and also an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers ...
, branched-chain paraffinic hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s (mostly isoheptane and isooctane). Alkylate is a premium gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
blending stock because it has exceptional antiknock properties and is clean burning. Alkylate is also a key component of avgas
Avgas (aviation gasoline, also known as aviation spirit in the United Kingdom, UK) is an aviation fuel used in aircraft with spark-ignited internal combustion engines. ''Avgas'' is distinguished from conventional gasoline (petrol) used in moto ...
. By combining fluid catalytic cracking, polymerization, and alkylation, refineries can obtain a gasoline yield of 70 percent. The widespread use of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
and hydrofluoric acid in refineries poses significant environmental risks. Ionic liquid
An ionic liquid (IL) is a salt (chemistry), salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as wate ...
s are used in place of the older generation of strong Bronsted acids.
Dealkylation
Complementing alkylation reactions are the reverse, dealkylations. Prevalent aredemethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen at ...
s, which are prevalent in biology, organic synthesis, and other areas, especially for methyl ethers and methyl amines.
See also
* Hydrodealkylation * Transalkylation * Alkynylation * Friedel–Crafts reaction * :Alkylating agents ** :Ethylating agents ** :Methylating agentsReferences
External links
Macrogalleria page on polycarbonate production
* {{Authority control Industrial processes Oil refining Organic reactions Chemical processes