|
Dimethyl Sulfate
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as ( CH3)2 SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis. Me2SO4 is a colourless oily liquid with a slight onion-like odour. Like all strong alkylating agents, Me2SO4 is toxic. Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid. History Impure dimethyl sulfate was prepared in the early 19th century. J. P. Claesson later extensively studied its preparation. It was investigated for possible use in chemical warfare in World War I in 75% to 25% mixture with methyl chlorosulfonate (CH3ClO3S) called "C-stoff" in Germany, or with chlorosulfonic acid called "Rationite" in France. The esterification of sulfuric acid with methanol was described in 1835: : Production Dimethyl s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver ( hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass animal testing, while maintaining the concept of toxicity endpoints. Etymology In Ancient Greek medical literature, the adjective ''τοξικόν'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylation
Methylation, in the chemistry, chemical sciences, is the addition of a methyl group on a substrate (chemistry), substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen#Compounds, hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology. In biological systems, methylation is Catalysis, catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of Protein#Functions, protein function, and RNA processing. ''In vitro'' methylation of tissue samples is also a way to reduce some histology#Histological Artifacts, histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function. It is a key pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
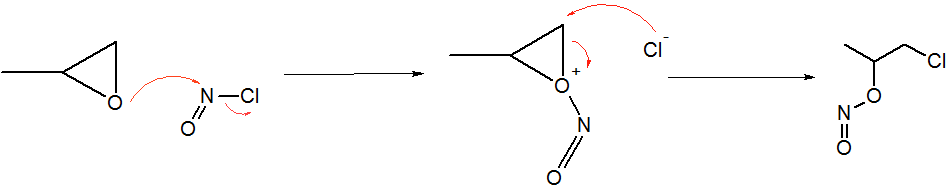
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Chlorosulfonate
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Nitrite
Methyl nitrite is an organic compound with the chemical formula . It is a gas, and is the simplest alkyl nitrite. Structure At room temperature, methyl nitrite exists as a mixture of ''cis'' and ''trans'' conformers. The ''cis'' conformer is 3.13 kJ mol−1, more stable than the ''trans'' form, with an energy barrier to rotation of 45.3 kJ mol−1. The cis and trans structure have also been determined by microwave spectroscopy (see external links). Synthesis Methyl nitrite can be prepared by the reaction of silver nitrite with iodomethane: Silver nitrite (AgNO2) exists in solution as the silver ion, Ag+ and the nitrite ion, NO2−. One of the lone pairs on an oxygen from nitrite ion attacks the methyl group (−CH3), releasing the iodide ion into solution. Unlike silver nitrite, silver iodide is highly insoluble in water and thus forms a solid. Note that nitrogen is a better nucleophile than oxygen and most nitrites would react via an SN2-like mechanism and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid. Sulfur trioxide exists in several forms: gaseous monomer, crystalline trimer, and solid polymer. Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain. Molecular structure and bonding Monomer The molecule SO3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). When the formal charge is non-zero, the S-O bonding is assumed to be delocalized. In any case the three S-O bond leng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3, (sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. Dimethyl ether was first synthesised by Jean-Baptiste Dumas and Eugene Péligot in 1835 by distillation of methanol and sulfuric acid. Production Approximately 50,000 tons were produced in 1985 in Western Europe by dehydration of methanol:Manfred Müller, Ute Hübsch, "Dimethyl Ether" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. : The required methanol is obtained from synthesis gas ( syngas). Other possible improvements call for a dual catalyst system that permits both methanol synthesis and dehydration in the same process unit, with no methanol isolation and purificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorosulfonic Acid
Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic and a powerful lachrymator. Commercial samples usually are pale brown or straw colored. Salts and esters of chlorosulfuric acid are known as chlorosulfates. Structure and properties Chlorosulfuric acid is a tetrahedral molecule. Its structure was debated for many decades until in 1941 Shrinivasa Dharmatti proved by magnetic susceptibility that chlorine is directly bonded to sulfur. The formula is more descriptively written SO2(OH)Cl, but HSO3Cl is traditional. It is an intermediate, chemically and conceptually, between sulfuryl chloride (SO2Cl2) and sulfuric acid (H2SO4). The compound is rarely obtained pure. Upon standing with excess sulfur trioxide, it decomposes to pyrosulfuryl chlorides: :2 ClSO3H + SO3 → H2SO4 + S2O5Cl2 Synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |