Nitrosyl chloride on:
[Wikipedia]
[Google]
[Amazon]
Nitrosyl chloride is the
 It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but i ...
, a mixture of 3 parts concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and 1 part of concentrated nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
. It is a strong electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
and oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.
Structure and synthesis
The molecule is bent. Adouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°.
Production
Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration ofnitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite () salts. It was discovered by Carl Wilhelm Scheele, who called it " phlogisticated acid of niter". Nitrous ac ...
by HCl
: HNO2 + HCl → H2O + NOCl
* By the direct combination of chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
; This reaction reverses above 100 °C.
: Cl2 + 2 NO → 2 NOCl
* By reduction of nitrogen dioxide with hydrogen chloride:
: 2NO2 + 4 HCl → 2NOCl + 2H2O + Cl2
Occurrence in aqua regia
NOCl also arises from the combination of hydrochloric and nitric acids according to the following reaction: :HNO3 + 3 HCl → 2 l+ 2 H2O + NOCl In nitric acid, NOCl is readily oxidized intonitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the s ...
. The presence of NOCl in aqua regia was described by Edmund Davy
Edmund Davy Fellow of the Royal Society, FRS (1785 – 5 November 1857)Christopher F. Lindsey, 'Davy, Edmund (1785–1857)’, Oxford Dictionary of National Biography, Oxford University Press, 200 accessed 6 April 2008/ref> was a professor of chemi ...
in 1831.
Reactions
NOCl behaves as an electrophile and an oxidant in most of its reactions. Withhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
acceptors it gives nitrosonium
The nitrosonium ion is , in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually o ...
salts, and synthesis of nitrosonium tetrachloroferrate is typically performed in liquid NOCl:
: NOCl + FeCl3 → Osup>+ eCl4sup>−
In a related reaction, sulfuric acid gives nitrosylsulfuric acid, the mixed acid anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of wa ...
of nitrous and sulfuric acid:
: ClNO + H2SO4 → ONHSO4 + HCl
NOCl reacts with silver thiocyanate to give silver chloride
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts ...
and the pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
nitrosyl thiocyanate:
: ClNO + AgSCN → AgCl + ONSCN
Similarly, it reacts with silver cyanide to give nitrosyl cyanide.
Nitrosyl chloride is used to prepare metal nitrosyl complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat complexes that contain nitric oxide">hypertension.
Metal nitrosyl complexes are complex (chemistry)">complexes that contain nitri ...
es. With molybdenum hexacarbonyl, NOCl gives the dinitrosyldichloride complex:
:Mo(CO)6 + 2 NOCl → MoCl2(NO)2 + 6 CO
It dissolves platinum:
:Pt + 6 NOCl → (NO+)2 tCl6sup>2- + 4 NO
Applications in organic synthesis
Aside from its role in the production of caprolactam, NOCl finds some other uses inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. It adds to alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s to afford α-chloro oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
s. The addition of NOCl follows the Markovnikov rule. Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
s also add NOCl, giving nitrosyl derivatives:
: H2C=C=O + NOCl → ONCH2C(O)Cl
Carbonyl compound
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ...
s enolize; and then NOCl attacks the nucleophilic end of the alkene to give a vicinal keto- or aldo-oxime.
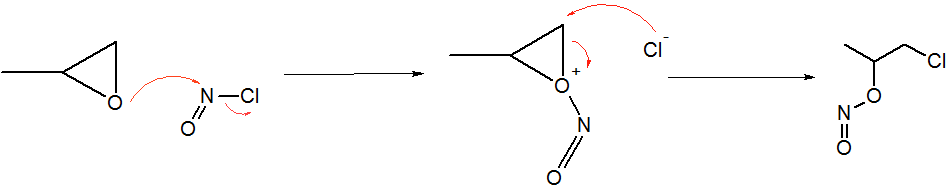
Epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s react with NOCl to give an α-chloronitritoalkyl derivatives. In the case of propylene oxide, the addition proceeds with high regiochemistry:
: It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example, aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its deriva ...
reacts with NOCl to give ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon double bonds).
Ethy ...
, nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
and hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
.
Industrial applications
NOCl andcyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
react photochemically to give cyclohexanone oxime hydrochloride. This process exploits the tendency of NOCl to undergo photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
into NO and Cl radicals. The cyclohexanone oxime is converted to caprolactam, a precursor to nylon-6.
Historical importance
Before the advent of modern spectroscopic methods for chemical analysis, informative chemical degradation and structure elucidation required the characterization of the individual components of various extracts. Notably, the aforementioned introduction of nitrosyl chloride by Tilden in 1875, as a reagent for producing crystalline derivatives of terpenes, e.g. α-pinene from oil of turpentine allowed investigators to readily distinguish one terpene from another.:Safety
Nitrosyl chloride is very toxic and irritating to the lungs, eyes, and skin.References
Bibliography
*External links
* {{ChloridesChloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
Oxychlorides
Nitrogen(III) compounds
Nitrogen oxohalides
Gases with color