Alkyl Ketene Dimer on:
[Wikipedia]
[Google]
[Amazon]
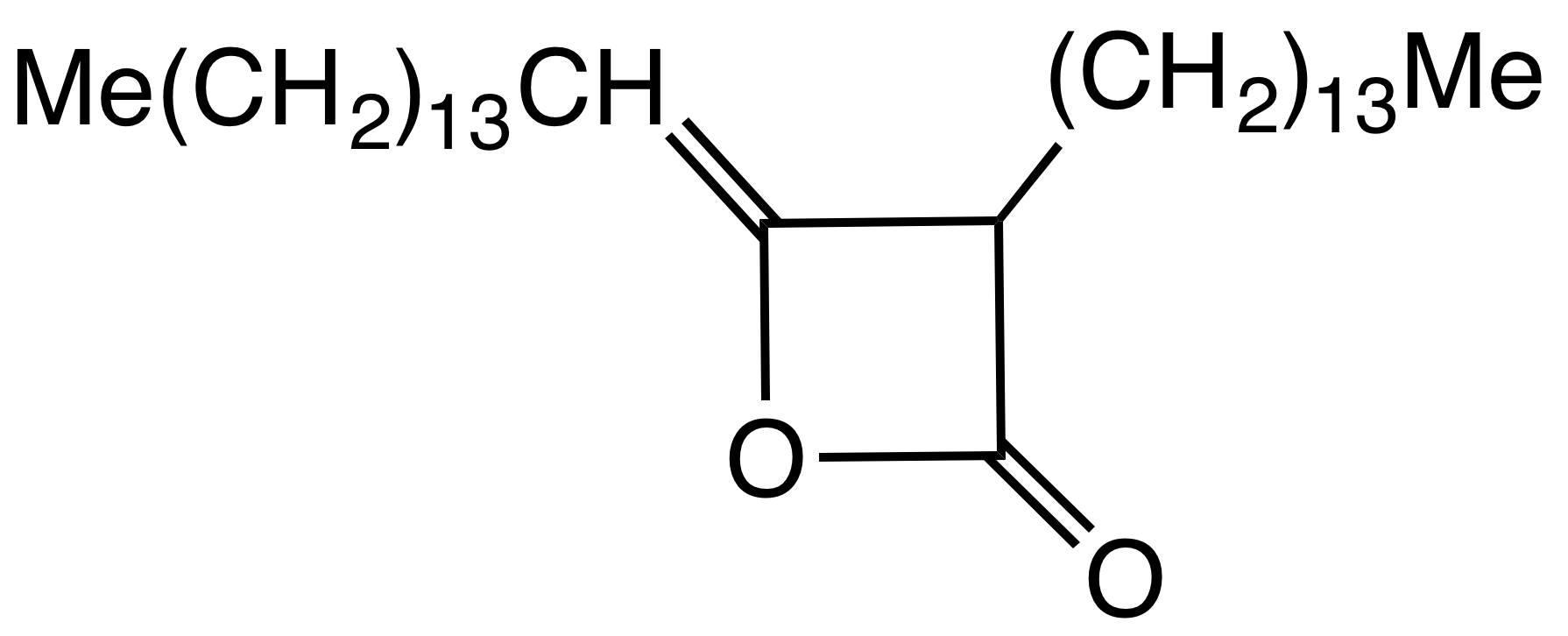 Alkyl ketene dimers (AKDs) are a family of
Alkyl ketene dimers (AKDs) are a family of
 The primary reaction products of acid chlorides with hydrogen atoms in α-position and tertiary amines were identified by
The primary reaction products of acid chlorides with hydrogen atoms in α-position and tertiary amines were identified by
 The use of other solvents, such as
The use of other solvents, such as
 Industrially applied AKDs are derived from fatty acids with chain lengths between C14 (
Industrially applied AKDs are derived from fatty acids with chain lengths between C14 (
 The molecular structure (i.e. molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
After removal of excess water - also to avoid hydrolysis of the AKD to the beta-keto acid and subsequent
The molecular structure (i.e. molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
After removal of excess water - also to avoid hydrolysis of the AKD to the beta-keto acid and subsequent  follows the cracking of the stabilized AKD particles on the base paper mass, the melting of the solid AKD wax (at approx. 90 °C), the spreading of the liquid AKD wax by
follows the cracking of the stabilized AKD particles on the base paper mass, the melting of the solid AKD wax (at approx. 90 °C), the spreading of the liquid AKD wax by
 Alkyl ketene dimers (AKDs) are a family of
Alkyl ketene dimers (AKDs) are a family of organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene
Diketene is an organic compound with the molecular formula , and which is sometimes written as . It is formed by dimerization of ketene, . Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorle ...
. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
in the 3-position and a C13 – C17 alkylidene group in the 4-position.
The main application of alkylated ketene dimers is in the sizing
Sizing or size is a substance that is applied to, or incorporated into, other materials—especially papers and textiles—to act as a protective filler or glaze. Sizing is used in papermaking and textile manufacturing to change the absorption ...
of paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
and cardboard
Cardboard is a generic term for heavy paper-based products. Their construction can range from a thick paper known as paperboard to corrugated fiberboard, made of multiple plies of material. Natural cardboards can range from grey to light brown ...
, as well as in the hydrophobation of cellulose fiber
Cellulose fibers () are fibers made with ethers or esters of cellulose, which can be obtained from the bark, wood or leaves of plants, or from other plant-based material. In addition to cellulose, the fibers may also contain hemicellulose and li ...
s. The products thus modified are distinguished by higher mechanical strengths and less penetration of water, ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. ...
s or printing ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. ...
s.
AKD's feature hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
alkyl groups extending from a beta-propiolactone ring. A specific example is derived from the dimerization of the ketene of stearic acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula . The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid ...
. This ketene is generated by pyrolysis of stearoyl chloride. AKD's react with the hydroxyl groups on the cellulose via esterification
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
reaction. The esterification is competitive with hydrolysis of the AKD. Prior to the development of AKD's, hydrophobicity was imparted by incorporating rosin
Rosin (), also known as colophony or Greek pitch (), is a resinous material obtained from pine trees and other plants, mostly conifers. The primary components of rosin are diterpenoids, i.e., C20 carboxylic acids. Rosin consists mainly of r ...
into the paper.
Related to AKDs, is alkenylsuccinic anhydride (ASA). As for AKDs, ASA reacts with hydroxy groups of the cellulose to form an ester, anchoring the hydrophobic group to the surface. ASA is prepared by the ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile) ...
of unsaturated hydrocarbons with maleic anhydride
Maleic anhydride is an organic compound with the formula . It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Str ...
.
History
In 1901,Edgar Wedekind
Edgar Leon Waldemar Otto Wedekind (31 January 1870 - 22 October 1938) was a German chemist and teacher at Hann. Münden, Hannoversch-Münden. He was one of the signatories for the ''Vow of allegiance of the Professors of the German Universities an ...
published the synthesis of alkyl ketene dimers by the reaction of carboxylic acid chlorides with tertiary amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
:
The molecular weight determined by the early researchers indicated )n where n > 1 for the dehydrohalogenation of isobutyryl chloride
Isobutyryl chloride (2-methylpropanoyl chloride) is the organic compound with the formula . A colorless liquid, it the simplest branched-chain acyl chloride. It is prepared by chlorination of isobutyric acid.
Reactions
As an ordinary acid chlor ...
with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
.
Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
and Norman Thomas Mortimer Wilsmore as highly reactive ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
s (ethenones) which form 2-oxetanones with an alkylidene group when dimerizing in a +2photocycloadditions.
Staudinger's insight was complicated by dependence of the dimerization on substituents. The simple ketene (H2C=C=O) dimerizes to diketene (4-methylen-oxetan-2-one), while substituted ketenes, such as dimethyl ketene (Me2C=C=O, formed from isobutyryl chloride with triethylamine) dimerize in a head-to-tail addition to 2,2,4,4-tetramethylcyclobutanedione
2,2,4,4-Tetramethylcyclobutanedione is the organic compound with the formula (CH3)4C4O2. The compound is a diketone of cyclobutane, bearing four methyl groups. It is a white solid that is used as a precursor to diverse industrial products.
Synth ...
.
The 2,2,4,4,4-tetramethylcyclobutanedione can easily be isomerized to dimethyl ketene dimer (4-isopropylidene-3,3-dimethyl-oxetan-2-one).
The synthesis and characterization of hexadecyl ketene dimer, a key substance for alkylated ketene dimers was first described in a patent in 1945 and in a publication in 1947.
Preparation
The industrial synthesis of alkylated ketenedimers (at that time still called ketoethenones) was patented in 1945 from long-chain carboxylic acid chlorides in inert solvents (such asdiethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
or benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
) with triethylamine as tertiary amine under anhydrous conditions. After filtration of the insoluble triethylamine hydrochloride and evaporation of the solvent, long-chain alkyl chain dimers are obtained in yields of more than 90%.
carboxylic acid ester
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
s or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s for easier separation of trialkylamine hydrochlorides or other amines, such as ''N,N,N',N-tetramethyl-hexane-1,6-diamine does not provide any significant advantages.
Also processes without the solvent use are described, in which the resulting amine hydrochloride is either filtered off or extracted with diluted aqueous acids.
A continuous process in which long-chain carboxylic acid chloride and tertiary amine (e. g. dimethyl isopropylamine, dimethylcyclohexylamine or triethylamine) is supplied separately without solvents to a tube reactor, kneader or preferably a twin-screw extruder or planetary roller extruder and reacted at temperatures between 90 and 110 °C, delivers lactone contents of over 90% at short reaction times. Processing is carried out by phase separation or acidic extraction.
Use
Alkylated ketene dimers as paper sizing agents
The problems with the acidic (aluminum sulfate-mediated) mass sizing of paper with alkaline-digested colophony resins introduced since the early 19th century led besides the use of alkaline flocculants (such aschalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Ch ...
or calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
as the alkali reserve) to the search for alternative materials for sizing in a neutral or alkaline environment. In addition to the significantly more reactive alkenylsuccinic anhydrides (which do also hydrolyze rapidly in the presence of water) alkylated ketene dimers have begun to be preferred surface and mass sizes in the paper industry from the 1960s onwards, beginning in the 1950s.
myristic acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula . Its salts and esters are commonly referred to as myristates or tetradecanoates. The name of the acyl group derived from myristic acid is m ...
) to C22 (behenic acid
Behenic acid (also docosanoic acid) is a carboxylic acid, the saturated fatty acid with formula . In appearance, it consists of white solid although impure samples appear yellowish.
Sources
At 9%, it is a major component of ben oil (or behen oi ...
); palmityl (C16) diketene and stearyl (C18) ketene and mixtures thereof are preferably used, as well as fatty acid mixtures from the hydrolysis of animal and vegetable fats. Because of the chain length of the original fatty acids, AKD are waxy solids with melting points between 42 and about 70 °C. Mixtures of alkylated ketene dimers and water are dispersions at temperatures below 40 °C or emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
s at temperatures above 45 °C. Liquid AKD are not widely used, they are based on unsaturated fatty acids like oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish due to the presence of impurities. In chemical terms, oleic acid is cl ...
or branched fatty acids, like isostearic acid.
Aqueous alkyldiketene dispersions generally contain 10-20 wt% of AKD, as well as active protective colloid
A protective colloid is a lyophilic colloid that when present in small quantities keeps lyophobic colloids from precipitating under the coagulating action of electrolytes.
Need for protective colloids
When a small amount of hydrophilic colloi ...
s (particularly polycations such as cationic starch, copolymers of ''N''-vinylpyrrolidone and quaternized ''N''-vinylimidazole, acylated polyethyleneimines or cationic high molecular weight polyacrylamides with an average molar mass up to 7 million g/mol) and other stabilizers (usually anionic surfactants, for example ligninsulfonates or condensation products of naphthalenesulfonic acid sodium salt and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
). Such stabilized AKD dispersions are active and stable at room temperature for up to three months and also tolerate the addition of different fillers for paper or cardboard (e.g. kaolin
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina (). ...
, chalk, talc, titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index Internationa ...
, calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
, aluminum oxide, etc.) from 5 to 25%. The amounts of alkyl ketene dimers used for the sizing of paper and paper products are preferably in the range from 0.15 to 0.8 wt%, sometimes from 0.05 to 0.2 wt%, based on the dry paper stock.
Paper sizing with alkylated ketene dimers
For paper sizing with AKD, a three-step process was proposed which, despite controversial discussions in the 1990s, seems to describe the processes that are taking place best and explains the results achieved. Decisive criteria for the quality of the hydrophobicity of papers are # the retention of the AKD particles on the wet paper mass on the paper screen # the spreading of the AKD particles on the surface and the penetration in the paper mass # the chemical reaction of thehydroxyl groups
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
of the cellulose (esterification
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
) with the alkylated ketene dimers to form beta-ketocarboxylic esters.
decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
to the ketone -
surface diffusion
Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters ( adparticles) at solid material surfaces.Oura, Lifshits, Saranin, Zotov, and Katayama 2003, p. 325 The process can generally be thought of in ...
on the cellulose fibers and the formation of closed hydrophobic layers. The thickness of the hydrophobic layers depends on the AKD concentration in the dispersion.
Ad 3. The hydrophobation of cellulose fibers with alkylated ketene dimers takes place most effectively in neutral or preferably weakly alkaline media (pH 7.5-9.0). The reaction temperature is generally 90-110 °C, with approximately 40% of the AKD used reacting with the cellulose. After the reaction contact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
s of >100° are measured, indicating the hydrophobic character of the AKD-modified model surfaces. The esterification of hydroxyl groups of cellulose fibers was also demonstrated by comparison reactions with 14C-labeled AKD.
The sizing with AKD is suitable for the permanent hydrophobation of newspaper, printing and writing paper and cardboard used as a container for liquids (including foodstuffs such as milk), as well as for the improvement of shape stability and runnability.
Literature
* * {{citation, first=D., last=Johnson, editor-surname1=I. Thorn, C.O. Au, title=Applications of Wet-End Paper Chemistry, 2nd Edition, publisher=Springer Netherlands, pages=73–112, isbn=978-1-4020-6037-3, date=2009References