|
Alkylidene Group
In organic chemistry, alkylidene is a general term for divalent functional groups of the form , where each R is an alkane or hydrogen. They can be considered the functional group corresponding to mono- or disubstituted divalent carbenes (known as alkylidenes), or as the result of removing two hydrogen atoms from the same carbon atom in an alkane. The simplest alkylidene group is the methylidene group, . This is also known by the common name methylene, which can also refer to the methylene bridge group or the diradical carbene . In organometallic chemistry, divalent ligands are referred to as carbenes, with the term "alkylidene" referring specifically to the narrower class of Schrock carbenes. Nomenclature In standard IUPAC nomenclature, alkylidene groups are named by replacing the -yl in the corresponding alkyl group with -ylidene. This practice is also often extended to common names. For example, the isopropyl group (IUPAC: prop-2-yl) corresponds to the isopropylidene group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present ( heterocyclic compounds with rings containing both carbon and non-carbon). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
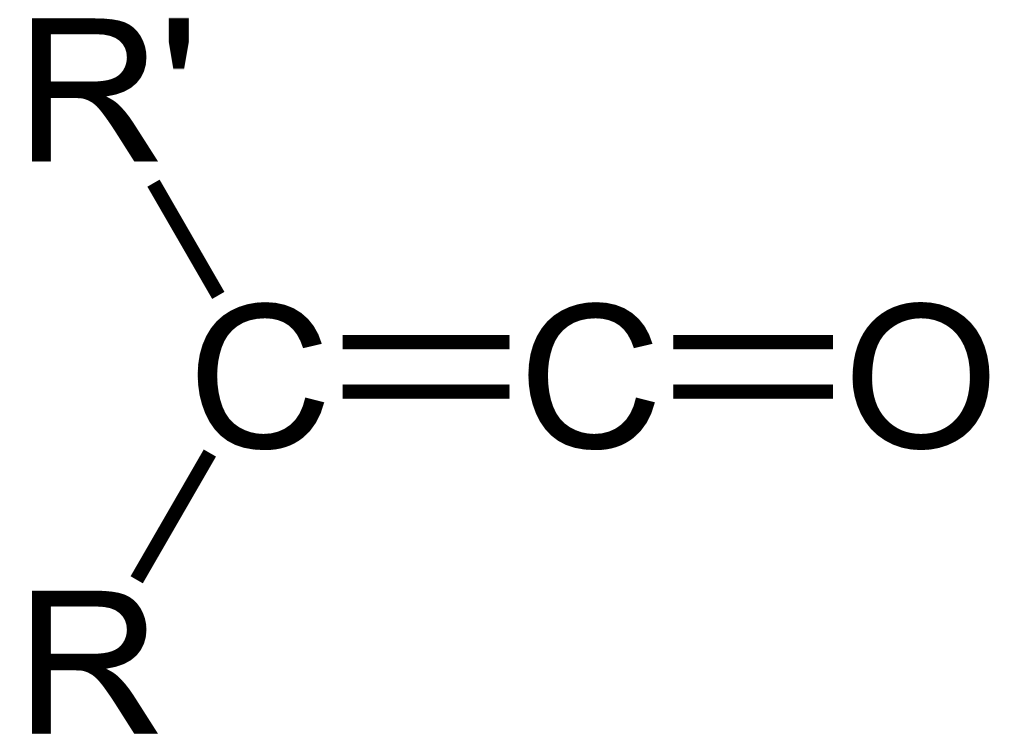
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonide
In organic chemistry, an acetonide is the functional group composed of the cyclic ketal of a diol with acetone. The more systematic name for this structure is an isopropylidene ketal. Acetonide is a common protecting group for 1,2- and 1,3-diols. The protecting group can be removed by hydrolysis of the ketal using dilute aqueous acid. Example The acetonides of small di- and triols, as well as many sugars and sugar alcohols, are common. The hexaol mannitol reacts with 2,2-dimethoxypropane to give the bis-acetonide, which oxidizes to give the acetonide of glyceraldehyde: :(CHOHCHOHCH2OH)2 + 2 (MeO)2CMe2 → (CHOHCHCH2O2CMe2)2 + 4 MeOH :(CHOHCHOCH2OCMe2)2 + → 2 OCHCHCH2O2CMe2 + H2O An example of its use as a protecting group in a complex organic synthesis is the Nicolaou Taxol total synthesis. It is a common protecting group for sugars and sugar alcohols, a simple example being solketal. The acetonides of corticosteroid are used in dermatology, because their increased lipop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is Hygroscopy, hygroscopic in nature. Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol. Structure Although chirality, achiral, glycerol is prochirality, prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the Glycerophospholipid#Nomenclature and stereochemistry, stereospecific numbering labels the molecule with a ''sn''- prefix before the stem name of the molecule. Production Natural sources Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meldrum's Acid
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula . Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as . It is a crystalline colorless solid that is sparingly soluble in water and which decomposes on heating to carbon dioxide, acetone, and a ketene. Its synthesis was first reported in 1908 by Andrew Norman Meldrum, for whom it is named. Meldrum incorrectly concluded that it was a carboxylic acid based on its acidity; the correct bislactone structure was not reported until 1948. Properties Acidity The compound can easily lose a hydrogen ion from the methylene () in the ring (carbon 5); which creates a double bond between it and one of the adjacent carbons (number 4 or 6), and a negative charge in the corresponding oxygen. The resulting anion is stabilized by resonance between the two alternatives, so that the double bond is delocalized and each oxygen in the carbonyls has a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solketal
Solketal is a protected form of glycerol with an isopropylidene acetal group joining two neighboring hydroxyl groups. Solketal contains a chiral center on the center carbon of the glycerol backbone, and so can be purchased as either the racemate or as one of the two enantiomers. Solketal has been used extensively in the synthesis of mono-, di- and triglycerides by ester bond formation. The free hydroxyl group of solketal can be esterified with a carboxylic acid to form the protected monoglyceride. The isopropylene group can then be removed using an acid catalyst in aqueous or alcoholic medium. The unprotected diol can then be esterified further to form either the di- or triglyceride. Another route to specific di- or triglycerides involves converting the solketal to glycidol (2,3-epoxy-1-propanol) and esterifying this with one fatty acid before opening the epoxy Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward creating polyethylene, which is a widely used plastic containing polymer chains of ethylene units in various chain lengths. Production greenhouse gas emissions, emits greenhouse gases, including methane from feedstock production and carbon dioxide from any non-sustainable energy used. Ethylene is also an important natural plant hormone and is used in agriculture to induce ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water (at a boiling point of ) and other chlorocarbons. History In 1794, physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt, under the name of Society of Dutch Chemists (), were the first to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas. Although the ''Gezelschap'' in practice did not do much in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |