Acyl on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 A major factor in determining the reactivity of acyl derivatives is leaving group ability, which is related to acidity. Weak bases are better leaving groups than strong bases; a species with a strong conjugate acid (e.g. hydrochloric acid) will be a better leaving group than a species with a weak conjugate acid (e.g.
A major factor in determining the reactivity of acyl derivatives is leaving group ability, which is related to acidity. Weak bases are better leaving groups than strong bases; a species with a strong conjugate acid (e.g. hydrochloric acid) will be a better leaving group than a species with a weak conjugate acid (e.g. 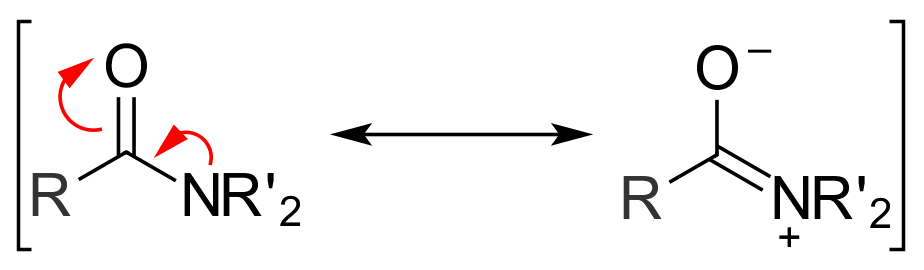 Another factor that plays a role in determining the reactivity of acyl compounds is
Another factor that plays a role in determining the reactivity of acyl compounds is
 Acylium ions are cations of the formula . The carbon–oxygen
Acylium ions are cations of the formula . The carbon–oxygen

 This mechanism is supported by isotope labeling experiments. When ethyl propionate with an oxygen-18-labeled ethoxy group is treated with
This mechanism is supported by isotope labeling experiments. When ethyl propionate with an oxygen-18-labeled ethoxy group is treated with 
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
and an organyl group () or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
in the case of formyl group (). In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the acyl group (IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
name alkanoyl if the organyl group is alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
) is usually derived from a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
, in which case it has the formula , where R represents an organyl group or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.
Reactivity trends
There are five main types of acyl derivatives. Acid halides are the most reactive towards nucleophiles, followed by anhydrides,ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, and amides. Carboxylate ions are essentially unreactive towards nucleophilic substitution, since they possess no leaving group. The reactivity of these five classes of compounds covers a broad range; the relative reaction rates of acid chlorides and amides differ by a factor of 1013.
: A major factor in determining the reactivity of acyl derivatives is leaving group ability, which is related to acidity. Weak bases are better leaving groups than strong bases; a species with a strong conjugate acid (e.g. hydrochloric acid) will be a better leaving group than a species with a weak conjugate acid (e.g.
A major factor in determining the reactivity of acyl derivatives is leaving group ability, which is related to acidity. Weak bases are better leaving groups than strong bases; a species with a strong conjugate acid (e.g. hydrochloric acid) will be a better leaving group than a species with a weak conjugate acid (e.g. acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
). Thus, chloride ion is a better leaving group than acetate ion. The reactivity of acyl compounds towards nucleophiles decreases as the basicity of the leaving group increases, as the table shows.Wade 2010, pp. 998–999.
 Another factor that plays a role in determining the reactivity of acyl compounds is
Another factor that plays a role in determining the reactivity of acyl compounds is resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
. Amides exhibit two main resonance forms. Both are major contributors to the overall structure, so much so that the amide bond between the carbonyl carbon and the amide nitrogen has significant double bond character. The energy barrier for rotation about an amide bond is 75–85 kJ/mol (18–20 kcal/mol), much larger than values observed for normal single bonds. For example, the C–C bond in ethane has an energy barrier of only 12 kJ/mol (3 kcal/mol). Once a nucleophile attacks and a tetrahedral intermediate is formed, the energetically favorable resonance effect is lost. This helps explain why amides are one of the least reactive acyl derivatives.
Esters exhibit less resonance stabilization than amides, so the formation of a tetrahedral intermediate and subsequent loss of resonance is not as energetically unfavorable. Anhydrides experience even weaker resonance stabilization, since the resonance is split between two carbonyl groups, and are more reactive than esters and amides. In acid halides, there is very little resonance, so the energetic penalty for forming a tetrahedral intermediate is small. This helps explain why acid halides are the most reactive acyl derivatives.
Compounds
Well-known acyl compounds are the acyl chlorides, such as acetyl chloride (CH3COCl) and benzoyl chloride (C6H5COCl). These compounds, which are treated as sources of acylium cations, are good reagents for attaching acyl groups to various substrates. Amides (RC(O)NR′2) andester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s (RC(O)OR′) are classes of acyl compounds, as are ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s (RC(O)R′) and aldehydes (RC(O)H), where R and R′ stand for organyl (or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
in the case of formyl).
Acylium cations, radicals, and anions
bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
in these cations is near 1.1 Å (110-112 pm), which is shorter than the 112.8 pm of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and indicates triple-bond character.
The carbon centres of acylium ions generally have a linear geometry and sp atomic hybridization, and are best represented by a resonance structure bearing a formal positive charge on the oxygen (rather than carbon): . They are characteristic fragments observed in EI- mass spectra of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s.
Acylium ions are common reactive intermediates, for example in the Friedel–Crafts acylation and many other organic reactions such as the Hayashi rearrangement. Salts containing acylium ions can be generated by removal of the halide from acyl halides:
:
Acyl radicals are readily generated from aldehydes by hydrogen-atom abstraction. However, they undergo rapid decarbonylation to afford the alkyl radical:
:
Acyl anions are almost always unstable—usually too unstable to be exploited synthetically. They readily react with the neutral aldehyde to form an acyloin dimer. Hence, synthetic chemists have developed various acyl anion synthetic equivalents, such as dithianes, as surrogates. However, as a partial exception, hindered dialkylformamides (e.g., diisopropylformamide, HCON''i''Pr2) can undergo deprotonation at low temperature (−78 °C) with lithium diisopropylamide as the base to form a carbamoyl anion stable at these temperatures.
In biochemistry
Inbiochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
there are many instances of acyl groups, in all major categories of biochemical molecules.
Acyl-CoAs are acyl derivatives formed via fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
metabolism. Acetyl-CoA, the most common derivative, serves as an acyl donor in many biosynthetic transformations. Such acyl compounds are thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
s.
Names of acyl groups of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s are formed by replacing the ''-ine'' suffix with ''-yl''. For example, the acyl group of glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
is glycyl, and of lysine is lysyl.
Names of acyl groups of ribonucleoside monophosphates such as AMP (5′-adenylic acid), GMP (5′-guanylic acid), CMP (5′-cytidylic acid), and UMP (5′-uridylic acid) are adenylyl, guanylyl, cytidylyl, and uridylyl respectively.
In phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s, the acyl group of phosphatidic acid is called phosphatidyl-.
Finally, many saccharide
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' m ...
s are acylated.
In organometallic chemistry and catalysis
Acylligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s are intermediates in many carbonylation reactions, which are important in some catalytic reactions. Metal acyls arise usually via insertion of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
into metal–alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
bonds. Metal acyls also arise from reactions involving acyl chlorides with low-valence metal complexes or by the reaction of organolithium compounds with metal carbonyls. Metal acyls are often described by two resonance structures, one of which emphasizes the basicity
In chemistry, there are three definitions in common use of the word "base": ''Arrhenius bases'', ''Brønsted bases'', and ''Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by Guilla ...
of the oxygen center. ''O''-alkylation of metal acyls gives Fischer carbene complexes.
Nomenclature
Thecommon name
In biology, a common name of a taxon or organism (also known as a vernacular name, English name, colloquial name, country name, popular name, or farmer's name) is a name that is based on the normal language of everyday life; and is often con ...
s of acyl groups are derived typically by replacing the -ic acid suffix of the corresponding carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
's common name with -yl (or -oyl), as shown in the table below.
In the IUPAC nomenclature of organic chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of O ...
, the systematic names of acyl groups are derived exactly by replacing the -yl suffix of the corresponding hydrocarbyl group's systemic name (or the -oic acid suffix of the corresponding carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
's systemic name) with -oyl, as shown in the table below.
The acyls are between the hydrocarbyls and the carboxylic acids.
The hydrocarbyl group names that end in -yl are not acyl groups, but alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
groups derived from alkanes ( methyl, ethyl, propyl, butyl), alkenyl groups derived from alkenes ( propenyl, butenyl), or aryl groups ( benzyl).
Reaction mechanisms
Acyl compounds react with nucleophiles via an addition mechanism: the nucleophile attacks the carbonyl carbon, forming a tetrahedral intermediate. This reaction can be accelerated byacid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ic conditions, which make the carbonyl more electrophilic, or basic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
conditions, which provide a more anionic and therefore more reactive nucleophile. The tetrahedral intermediate itself can be an alcohol or alkoxide, depending on the pH of the reaction.
The tetrahedral intermediate of an acyl compound contains a substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
attached to the central carbon that can act as a leaving group. After the tetrahedral intermediate forms, it collapses, recreating the carbonyl C=O bond and ejecting the leaving group in an elimination reaction. As a result of this two-step addition/elimination process, the nucleophile takes the place of the leaving group on the carbonyl compound by way of an intermediate state that does not contain a carbonyl. Both steps are reversible and as a result, nucleophilic acyl substitution reactions are equilibrium processes.Wade 2010, pp. 996–997. Because the equilibrium will favor the product containing the best nucleophile, the leaving group must be a comparatively poor nucleophile in order for a reaction to be practical.
Acidic conditions
Under acidic conditions, the carbonyl group of the acyl compound 1 is protonated, which activates it towards nucleophilic attack. In the second step, the protonated carbonyl 2 is attacked by a nucleophile (H−Z) to give tetrahedral intermediate 3. Proton transfer from the nucleophile (Z) to the leaving group (X) gives 4, which then collapses to eject the protonated leaving group (H−X), giving protonated carbonyl compound 5. The loss of a proton gives the substitution product, 6. Because the last step involves the loss of a proton, nucleophilic acyl substitution reactions are considered catalytic in acid. Also note that under acidic conditions, a nucleophile will typically exist in its protonated form (i.e. H−Z instead of Z−). :
Basic conditions
Underbasic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
conditions, a nucleophile (Nuc) attacks the carbonyl group of the acyl compound 1 to give tetrahedral alkoxide intermediate 2. The intermediate collapses and expels the leaving group (X) to give the substitution product 3. While nucleophilic acyl substitution reactions can be base-catalyzed, the reaction will not occur if the leaving group is a stronger base than the nucleophile (i.e. the leaving group must have a higher p''K''a than the nucleophile). Unlike acid-catalyzed processes, both the nucleophile and the leaving group exist as anions under basic conditions.
: This mechanism is supported by isotope labeling experiments. When ethyl propionate with an oxygen-18-labeled ethoxy group is treated with
This mechanism is supported by isotope labeling experiments. When ethyl propionate with an oxygen-18-labeled ethoxy group is treated with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
(NaOH), the oxygen-18 label is completely absent from propionic acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a ...
and is found exclusively in the ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
.
:
Acyl species
In acyloxy groups the acyl group is bonded to oxygen: R−C(=O)−O−R′ where R−C(=O) is the acyl group. Acylium ions are cations of the formula R−C≡O+. They are intermediates in Friedel-Crafts acylations.See also
* Acylation *Functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
References
External links
* {{Authority control Functional groups