Acetic Acid Ester on:
[Wikipedia]
[Google]
[Amazon]
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and
Chapter P-12.1; page 4
/ref> The name "acetic acid" derives from the
 In 1845 German chemist
In 1845 German chemist



 Acetic acid is produced industrially both naturally via bacterial
Acetic acid is produced industrially both naturally via bacterial
 The process involves
The process involves
National Pollutant Inventory – Acetic acid fact sheetMethod for sampling and analysis29 CFR 1910.1000, Table Z-1
(US Permissible exposure limits)
*Calculation o
vapor pressureliquid densitydynamic liquid viscositysurface tension
of acetic acid
Acetic acid bound to proteins
in the Protein Data Bank, PDB
Swedish Chemicals Agency. Information sheet – Acetic Acid
* Process Flow sheet of Acetic acid Production by th
Carbonylation of Methanol
{{Authority control Acetic acids, Acids in wine Antiseptics Commodity chemicals E-number additives Flavors Household chemicals Otologicals Photographic chemicals Solvents World Health Organization essential medicines Organic compounds with 2 carbon atoms Acetyl compounds
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
(also written as , , or ). Vinegar
Vinegar () is an aqueous solution of diluted acetic acid and trace compounds that may include flavorings. Vinegar typically contains from 5% to 18% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting ...
is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities.
Acetic acid is the second simplest carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
(after formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
). It is an important chemical reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
and industrial chemical across various fields, used primarily in the production of cellulose acetate
In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and ...
for photographic film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin photographic emulsion, emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the ...
, polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue (a term that may also refer to other types of glues), PVA glue, white glue, carpenter's glue, school glue, or Elmer's Glue in the US, is a widely available adh ...
for wood glue
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantage ...
, and synthetic fibres
Synthetic fibers or synthetic fibres (in British English; see spelling differences) are fibers made by humans through chemical synthesis, as opposed to natural fibers that are directly derived from living organisms, such as plants like cotton or ...
and fabrics. In households, diluted acetic acid is often used in descaling agent
A descaling agent or chemical descaler is a liquid chemical substance used to remove limescale from metal surfaces in contact with hot water, such as in boilers, water heaters, and kettles. Limescale is either white or brown in colour due to ...
s. In the food industry
The food industry is a complex, global network of diverse businesses that supplies most of the food consumed by the world's population. The food industry today has become highly diversified, with manufacturing ranging from small, traditional, ...
, acetic acid is controlled by the food additive code E260 as an acidity regulator
Acidity regulators, or pH control agents, are food additives used to change or maintain pH ( acidity or basicity). They can be organic or mineral acids, bases, neutralizing agents, or buffering agents. Typical agents include the following ac ...
and as a condiment. In biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, the acetyl group
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
, it is central to the metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
of carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s and fat
In nutrition science, nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such chemical compound, compounds, most commonly those that occur in living beings or in food.
The term often refers specif ...
s.
The global demand for acetic acid as of 2023 is about 17.88 million metric tonnes
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the shor ...
per year (t/a). Most of the world's acetic acid is produced via the carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
. Its production and subsequent industrial use poses health hazards to workers, including incidental skin damage and chronic respiratory injuries from inhalation.
Nomenclature
Thetrivial name
In chemistry, a trivial name is a non-systematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC inorganic or IUPAC organic nomenclature. A ...
"acetic acid" is the most commonly used and preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
. The systematic name "ethanoic acid", a valid IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
name, is constructed according to the substitutive nomenclature.IUPAC Provisional Recommendations 200Chapter P-12.1; page 4
/ref> The name "acetic acid" derives from the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
word for vinegar
Vinegar () is an aqueous solution of diluted acetic acid and trace compounds that may include flavorings. Vinegar typically contains from 5% to 18% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting ...
, "", which means "to sour" in relation to the bitter taste of fermented fruits.
"Glacial acetic acid" is a name for water-free (anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
) acetic acid. Similar to the German
German(s) may refer to:
* Germany, the country of the Germans and German things
**Germania (Roman era)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizenship in Germany, see also Ge ...
name "Eisessig" ("ice vinegar"), the name comes from the solid ice-like crystals that form with agitation, slightly below room temperature at . Acetic acid can never be truly water-free in an atmosphere that contains water, so the presence of 0.1% water in glacial acetic acid lowers its melting point by 0.2 °C.
A common symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
for acetic acid is AcOH (or HOAc), where Ac is the pseudoelement symbol
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an organic compound is a type of minimalist structural formula representing a molecule's atoms, bonds and some details of its geometry. The lines in a skeleta ...
representing the acetyl
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
; the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
, acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
(), is thus represented as . Acetate is the ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
resulting from loss of from acetic acid. The name "acetate" can also refer to a salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
containing this anion, or an ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
of acetic acid. (The symbol Ac for the acetyl functional group is not to be confused with the symbol Ac for the element actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
; context prevents confusion among organic chemists). To better reflect its structure, acetic acid is often written as , , , and . In the context of acid–base reaction
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms an ...
s, the abbreviation HAc is sometimes used, where Ac in this case is a symbol for acetate (rather than acetyl).
The carboxymethyl functional group derived from removing one hydrogen from the methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
group of acetic acid has the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
.
History
Vinegar
Vinegar () is an aqueous solution of diluted acetic acid and trace compounds that may include flavorings. Vinegar typically contains from 5% to 18% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting ...
is a diluted solution of acetic acid and was known early in civilization as the natural result of exposure of beer
Beer is an alcoholic beverage produced by the brewing and fermentation of starches from cereal grain—most commonly malted barley, although wheat, maize (corn), rice, and oats are also used. The grain is mashed to convert starch in the ...
and wine
Wine is an alcoholic drink made from Fermentation in winemaking, fermented fruit. Yeast in winemaking, Yeast consumes the sugar in the fruit and converts it to ethanol and carbon dioxide, releasing heat in the process. Wine is most often made f ...
to air because acetic acid-producing bacteria are present globally. It is likely the first acid to be produced in large quantities in history. The use of acetic acid in alchemy
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
extends into the third century BC, when the Greek philosopher Theophrastus
Theophrastus (; ; c. 371 – c. 287 BC) was an ancient Greek Philosophy, philosopher and Natural history, naturalist. A native of Eresos in Lesbos, he was Aristotle's close colleague and successor as head of the Lyceum (classical), Lyceum, the ...
described how vinegar acted on metals to produce pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
s useful in art, including ''white lead'' (lead carbonate
Lead(II) carbonate is the chemical compound with the chemical formula . It is a white, toxic solid. It occurs naturally as the mineral cerussite.
Structure
Like all metal carbonates, lead(II) carbonate adopts a dense, highly crosslinked structure ...
) and ''verdigris
Verdigris () is a common name for any of a variety of somewhat toxic copper salt (chemistry), salts of acetic acid, which range in colour from green to a blue-green, bluish-green depending on their chemical composition.H. Kühn, Verdigris and Cop ...
'', a green mixture of copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
salts including copper(II) acetate
Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula where is acetate (). The hydrated derivative, , which contains one molecule of water for each copper atom, is available commercially. Anhydrous co ...
. Hippocrates used vinegar as an antiseptic and as a remedy for numerous conditions including fever, constipation, ulcers, and pleurisy. Ancient Romans
Roman or Romans most often refers to:
*Rome, the capital city of Italy
*Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of Roman civilization
*Epistle to the Romans, shortened to Romans, a letter w ...
boiled soured wine to produce a highly sweet syrup called ''sapa''. Sapa
Sapa or Sapë may refer to:
Places
* Sapa, Mississippi, a community in the United States
* Sa Pa, a district-level town in Lào Cai province, Northern Vietnam
** Sa Pa (ward)
** Sa Pa (ward), Sa Pả (ward)
* Sapë, a town in Albania
* Roman Cath ...
that was produced in lead pots was rich in lead acetate
Lead () is a chemical element; it has symbol Pb (from Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lea ...
, a sweet substance also called ''sugar of lead'' or ''sugar of Saturn
Saturn is the sixth planet from the Sun and the second largest in the Solar System, after Jupiter. It is a gas giant, with an average radius of about 9 times that of Earth. It has an eighth the average density of Earth, but is over 95 tim ...
'', which contributed to lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, numbness and paresthesia, t ...
among the Roman aristocracy.
In the 16th-century German
German(s) may refer to:
* Germany, the country of the Germans and German things
**Germania (Roman era)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizenship in Germany, see also Ge ...
alchemist Andreas Libavius
Andreas Libavius or Andrew Libavius was born in Halle, Germany and died in July 1616. Libavius was a renaissance man who spent time as a professor at the University of Jena teaching history and poetry. After which he became a physician at the ...
described the production of acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
from the dry distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be ...
of lead acetate, ketonic decarboxylation
Ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction involving decarboxylation, converting two equivalents of a carboxylic acid () to a symmetric ketone (). The reaction typically requires heat and a ...
. The presence of water in vinegar has such a profound effect on acetic acid's properties that for centuries chemists believed that glacial acetic acid and the acid found in vinegar were two different substances. French chemist Pierre Adet
Pierre-Auguste, chevalier Adet (; 17 May 1763 Nevers – 19 March 1834 Paris) was a French scientist, politician, and diplomat.
He worked with Lavoisier on a new chemical notation system, and was secretary to the scientific periodical ''Annales d ...
proved them identical.
 In 1845 German chemist
In 1845 German chemist Hermann Kolbe
Adolph Wilhelm Hermann Kolbe (27 September 1818 – 25 November 1884) was a German chemist and academic, and a major contributor to the birth of modern organic chemistry. He was a professor at Marburg and Leipzig. Kolbe was the first to apply t ...
synthesized acetic acid from inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
s for the first time. This reaction sequence consisted of chlorination of carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
to carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
, followed by pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
to tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liqu ...
and aqueous chlorination to trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are cal ...
, and concluded with electrolytic
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble salts, acids, and bases, dissolved in a polar solvent like water. Upon dissolving, the ...
reduction to acetic acid.
By 1910, most glacial acetic acid was obtained from the pyroligneous liquor, a product of the distillation of wood. The acetic acid was isolated by treatment with milk of lime, and the resulting calcium acetate
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous ...
was then acidified with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
to recover acetic acid. At that time, Germany was producing 10,000 ton
Ton is any of several units of measure of mass, volume or force. It has a long history and has acquired several meanings and uses.
As a unit of mass, ''ton'' can mean:
* the '' long ton'', which is
* the ''tonne'', also called the ''metric ...
s of glacial acetic acid, around 30% of which was used for the manufacture of indigo dye
Indigo dye is an organic compound with a distinctive indigo, blue color. Indigo is a natural dye obtained from the leaves of some plants of the Indigofera#Uses, ''Indigofera'' genus, in particular ''Indigofera tinctoria''. Dye-bearing ''Indigofer ...
.
Because both methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
are commodity raw materials, methanol carbonylation long appeared to be attractive precursors to acetic acid. Henri Dreyfus
Henri Dreyfus (or Henry Dreyfus, 7 January 1882 – 30 December 1944) was a Switzerland, Swiss Invention, inventor of the modern Loom, weaving loom. He and his brother Camille Dreyfus (chemist), Camille Dreyfus also invented Celanese, an acetate y ...
at British Celanese
British Celanese was a chemical company based in England. Formed in 1916, it survived as an independent company until 1957 when it became a subsidiary of Courtaulds.
History
The origins of the company lie with two brothers, Henri and Camille ...
developed a methanol carbonylation pilot plant as early as 1925. However, a lack of practical materials that could contain the corrosive reaction mixture at the high pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
s needed (200 atm or more) discouraged commercialization of these routes. The first commercial methanol carbonylation process, which used a cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
catalyst, was developed by German chemical company BASF
BASF SE (), an initialism of its original name , is a European Multinational corporation, multinational company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters are located in Ludwigshafen, Ge ...
in 1963. In 1968, a rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
-based catalyst (''cis''−) was discovered that could operate efficiently at lower pressure with almost no by-products. US chemical company Monsanto Company
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best-known product is Roundup, a glyphosate-based herbicide, developed in ...
built the first plant using this catalyst in 1970, and rhodium-catalyzed methanol carbonylation became the dominant method of acetic acid production (see Monsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Che ...
). In the late 1990s, BP Chemicals commercialized the Cativa catalyst (), which is promoted by iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
for greater efficiency. Known as the Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
, the iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
-catalyzed production of glacial acetic acid is greener, and has largely supplanted the Monsanto process, often in the same production plants.
Interstellar medium
Interstellar acetic acid was discovered in 1996 by a team led by David Mehringer using the former Berkeley-Illinois-Maryland Association array at theHat Creek Radio Observatory
The Hat Creek Radio Observatory (HCRO) is operated by the SETI Institute in the Western United States. The observatory is home to the Allen Telescope Array and one of the three CHIME FRB outriggers, as well a number of other smaller telescopes ...
and the former Millimeter Array
The Atacama Large Millimeter/submillimeter Array (ALMA) is an astronomical interferometer of 66 radio telescopes in the Atacama Desert of northern Chile, which observe electromagnetic radiation at millimeter and submillimeter wavelengths. The arr ...
located at the Owens Valley Radio Observatory
Owens Valley Radio Observatory (OVRO) is a radio astronomy observatory located near Big Pine, California (US) in Owens Valley. It lies east of the Sierra Nevada, approximately north of Los Angeles and southeast of Bishop. It was established in 19 ...
. It was first detected in the Sagittarius B2
Sagittarius B2 (Sgr B2) is a giant molecular cloud of gas and dust that is located about from the center of the Milky Way. This complex is the largest molecular cloud in the vicinity of the core and one of the largest in the galaxy, spanning a re ...
North molecular cloud (also known as the Sgr B2 Large Molecule Heimat
The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine
Glycine (symbol Gly or G; ) is an amino acid that has a ...
source). Acetic acid has the distinction of being the first molecule discovered in the interstellar medium using solely radio interferometers; in all previous ISM molecular discoveries made in the millimetre and centimetre wavelength regimes, single dish radio telescopes were at least partly responsible for the detections.
Properties

Acidity
The hydrogen centre in thecarboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
(−COOH) in carboxylic acids such as acetic acid can separate from the molecule by ionization:
:
Because of this release of the proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
(), acetic acid has acidic character. Acetic acid is a weak monoprotic acid. In aqueous solution, it has a pKa value of 4.76. Its conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
is acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
(). A 1.0 M solution (about the concentration of domestic vinegar) has a pH of 2.4, indicating that merely 0.4% of the acetic acid molecules are dissociated.
Structure
In solid acetic acid, the molecules form chains of individual molecules interconnected byhydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s. In the vapour phase at , dimers can be detected. Dimers also occur in the liquid phase in dilute solutions with non-hydrogen-bonding solvents, and to a certain extent in pure acetic acid, but are disrupted by hydrogen-bonding solvents. The dissociation enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
of the dimer is estimated at 65.0–66.0 kJ/mol, and the dissociation entropy at 154–157 J mol−1 K−1. Other carboxylic acids engage in similar intermolecular hydrogen bonding interactions.
Solvent properties
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
acetic acid is a hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
(polar
Polar may refer to:
Geography
* Geographical pole, either of the two points on Earth where its axis of rotation intersects its surface
** Polar climate, the climate common in polar regions
** Polar regions of Earth, locations within the polar circ ...
) protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labi ...
, similar to ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
. With a relative static permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insula ...
(dielectric constant) of 6.2, it dissolves not only polar compounds such as inorganic salts and sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s, but also non-polar compounds such as oils as well as polar solutes. It is miscible with polar and non-polar solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s such as water, chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
, and hexane
Hexane () or ''n''-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
Hexane is a colorless liquid, odorless when pure, and with a boiling point of approximately . It is widely used as ...
. With higher alkanes (starting with octane
Octane is a hydrocarbon and also an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers ...
), acetic acid is not miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically ...
at all compositions, and solubility of acetic acid in alkanes declines with longer n-alkanes. The solvent and miscibility
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically ...
properties of acetic acid make it a useful industrial chemical, for example, as a solvent in the production of dimethyl terephthalate
Dimethyl terephthalate (DMT) is an organic compound with the formula . It is the ester, diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.
Production
Dimethyl terephthalat ...
.
Biochemistry
At physiological pHs, acetic acid is usually fully ionized toacetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
in aqueous solution.
The acetyl
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
, formally derived from acetic acid, is fundamental to all forms of life. Typically, it is bound to coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
by acetyl-CoA synthetase
Acetyl-CoA synthetase (ACS), also commonly known as Acetate—CoA ligase or acetyl activating enzyme, is an enzyme () of the ligase class of enzymes which catalyze the formation of new chemical bonds between two molecules. As such, it is involved ...
enzymes, where it is central to the metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
of carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s and fat
In nutrition science, nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such chemical compound, compounds, most commonly those that occur in living beings or in food.
The term often refers specif ...
s. Unlike longer-chain carboxylic acids (the fatty acids
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
), acetic acid does not occur in natural triglyceride
A triglyceride (from '' tri-'' and '' glyceride''; also TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids.
Triglycerides are the main constituents of body fat in humans and other vertebrates ...
s. Most of the acetate generated in cells for use in acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, o ...
is synthesized directly from ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
or pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
. However, the artificial triglyceride triacetin
Triacetin is the organic compound with the formula . It is classified as a triglyceride, i.e., the triester of glycerol with acetic acid. It is a colorless, viscous, and odorless liquid with a high boiling point and a low melting point. It has a ...
(glycerine triacetate) is a common food additive and is found in cosmetics and topical medicines; this additive is metabolized to glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
and acetic acid in the body.
Acetic acid is produced and excreted
Excretion is elimination of metabolic waste, which is an essential process in all organisms. In vertebrates, this is primarily carried out by the lungs, kidneys, and skin. This is in contrast with secretion, where the substance may have specific ...
by acetic acid bacteria
Acetic acid bacteria (AAB) are a group of Gram-negative bacteria which Oxidation, oxidize sugars or ethanol and produce acetic acid during Aerobic fermentation, fermentation. The acetic acid bacteria consist of 10 genus, genera in the family Acet ...
, notably the genus ''Acetobacter
''Acetobacter'' is a genus of acetic acid bacteria. Acetic acid bacteria are characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. Of these, the genus ''Acetobacter'' is distinguished by the ability to oxidiz ...
'' and ''Clostridium acetobutylicum
''Clostridium acetobutylicum'', ATCC 824, is a commercially valuable bacterium sometimes called the "Weizmann Organism", after Jewish Russian-born biochemist Chaim Weizmann. A senior lecturer at the University of Manchester, England, he used th ...
''. These bacteria are found universally in food
Food is any substance consumed by an organism for Nutrient, nutritional support. Food is usually of plant, animal, or Fungus, fungal origin and contains essential nutrients such as carbohydrates, fats, protein (nutrient), proteins, vitamins, ...
stuffs, water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, and soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
, and acetic acid is produced naturally as fruits and other foods spoil. Acetic acid is also a component of the vaginal lubrication
Vaginal lubrication is a naturally produced fluid that lubricates the vagina. Vaginal lubrication production increases significantly during sexual arousal in anticipation of sexual intercourse. Vaginal dryness is the condition in which this lub ...
of human
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
s and other primate
Primates is an order (biology), order of mammals, which is further divided into the Strepsirrhini, strepsirrhines, which include lemurs, galagos, and Lorisidae, lorisids; and the Haplorhini, haplorhines, which include Tarsiiformes, tarsiers a ...
s, where it appears to serve as a mild antibacterial
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention ...
agent.
Production
 Acetic acid is produced industrially both naturally via bacterial
Acetic acid is produced industrially both naturally via bacterial fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
and synthetically. About 75% of acetic acid made for use in the chemical industry is made by the carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
, explained below. The biological route accounts for only about 10% of world production, but it remains important for the production of vinegar because many food purity laws require vinegar used in foods to be of biological origin. Other processes are methyl formate
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colourless liquid with an ethereal odour, high vapor pressure, and low surface tension. The gas form is odourl ...
isomerization, conversion of syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
to acetic acid, and gas phase oxidation of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
.
Acetic acid can be purified via fractional freezing
Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals
A metal ( ...
using an ice bath. The water and other impurities
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid. They differ from the chemical composition of the material or compound. Firstly, a pure chemical should appear in at least on ...
will remain liquid while the acetic acid will precipitate
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemic ...
out. As of 2003–2005, total worldwide production of virgin acetic acid was estimated at 5 Mt/a (million tonnes per year), approximately half of which was produced in the United States. European production was approximately 1 Mt/a and declining, while Japanese production was 0.7 Mt/a. Another 1.5 Mt were recycled each year, bringing the total world market to 6.5 Mt/a. Since then, the global production has increased from 10.7 Mt/a in 2010 to 17.88 Mt/a in 2023. The two biggest producers of virgin acetic acid are Celanese
Celanese Corporation, formerly known as Hoechst Celanese, is an American technology and specialty materials company headquartered in Irving, Texas. It is a Fortune 500 corporation. The company is the world's leading producer of acetic acid, pr ...
and BP Chemicals. Other major producers include Millennium Chemicals
Millennium Inorganic Chemicals is a Hunt Valley, Maryland based chemical company.
The business was established in 1985. It was a subsidiary of British conglomerate Hanson plc at one time, but was demerged on 1 October 1996, when it became an i ...
, Sterling Chemicals, Samsung
Samsung Group (; stylised as SΛMSUNG) is a South Korean Multinational corporation, multinational manufacturing Conglomerate (company), conglomerate headquartered in the Samsung Town office complex in Seoul. The group consists of numerous a ...
, Eastman, and .
Methanol carbonylation
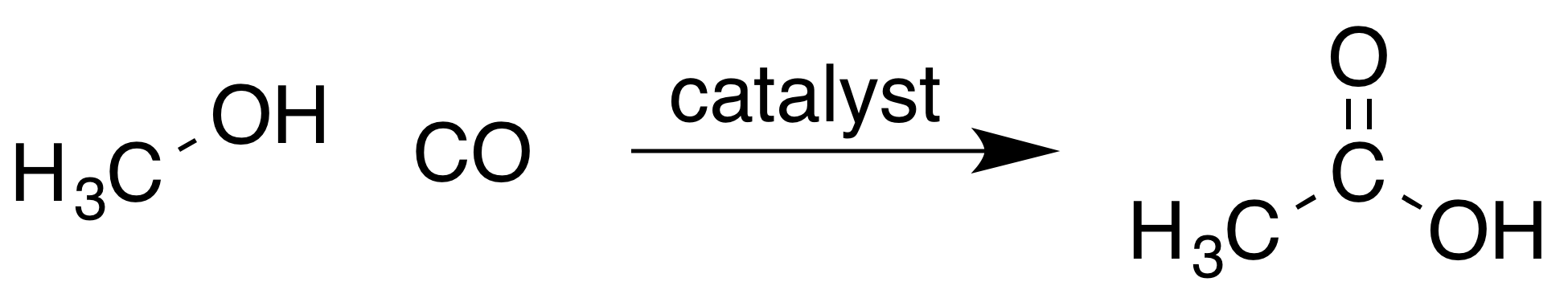
Most acetic acid is produced by methanolcarbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
. In this process, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
react to produce acetic acid according to the equation:
: The process involves
The process involves iodomethane
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hy ...
as an intermediate, and occurs in three steps. A metal carbonyl
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against n ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
is needed for the carbonylation (step 2).
#
#
#
Two related processes exist for the carbonylation of methanol: the rhodium-catalyzed Monsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Che ...
, and the iridium-catalyzed Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
. The latter process is greener and more efficient and has largely supplanted the former process. Catalytic amounts of water are used in both processes, but the Cativa process requires less, so the water-gas shift reaction
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of the fuel produced by this method is about 10% o ...
is suppressed, and fewer by-products are formed.
By altering the process conditions, acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
may also be produced in plants using rhodium catalysis.
Acetaldehyde oxidation
Prior to the commercialization of the Monsanto process, most acetic acid was produced by oxidation ofacetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
. This remains the second-most-important manufacturing method, although it is usually not competitive with the carbonylation of methanol. The acetaldehyde can be produced by hydration of acetylene. This was the dominant technology in the early 1900s.
Light naphtha
Naphtha (, recorded as less common or nonstandard in all dictionaries: ) is a flammable liquid hydrocarbon mixture. Generally, it is a fraction of crude oil, but it can also be produced from natural-gas condensates, petroleum distillates, and ...
components are readily oxidized by oxygen or even air to give peroxides
In chemistry, peroxides are a group of compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and ...
, which decompose to produce acetic acid according to the chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side ...
, illustrated with butane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at ro ...
:
:
Such oxidations require metal catalyst, such as the naphthenate
Naphthenic acids (NAs) are mixtures of several Cyclopentane, cyclopentyl and Cyclohexane, cyclohexyl carboxylic acids with molecular weights of 120 to well over 700 atomic mass units. The main Fraction (chemistry), fractions are carboxylic acids wi ...
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
s of manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
, and chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
.
The typical reaction is conducted at temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
s and pressures designed to be as hot as possible while still keeping the butane a liquid. Typical reaction conditions are and 55 atm. Side-products may also form, including butanone
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large s ...
, ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
, and propionic acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a ...
. These side-products are also commercially valuable, and the reaction conditions may be altered to produce more of them where needed. However, the separation of acetic acid from these by-products adds to the cost of the process.
Similar conditions and catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s are used for butane oxidation, the oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
to produce acetic acid can oxidize acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
.
:
Using modern catalysts, this reaction can have an acetic acid yield greater than 95%. The major side-products are ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
, and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, all of which have lower boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s than acetic acid and are readily separated by distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
.
Ethylene oxidation
Acetaldehyde may be prepared fromethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
via the Wacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) is an industrial chemical reaction: the aerobic oxidation of ethylene to acetaldehyde in the presence of catalysis, catalytic, aqueous palladium( ...
, and then oxidized as above.
In more recent times, chemical company Showa Denko
{{Infobox company
, name = Resonac K.K.
, native_name = レゾナック株式会社
, native_name_lang = ja
, romanized_name = Rezonakku kabushiki gaisha
, logo = Resonac logo.svg
, type = Public
, traded_as = {{tyo, 4004
, hq_location_city ...
, which opened an ethylene oxidation plant in Ōita, Japan, in 1997, commercialized a cheaper single-stage conversion of ethylene to acetic acid. The process is catalyzed by a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
metal catalyst supported on a heteropoly acid
The heteropolymetalates are a subset of the polyoxometalates, which consist of three or more transition metal oxyanions linked together by shared oxygen atoms to form a closed 3-dimensional molecular framework. In contrast to isopolymetalates, wh ...
such as silicotungstic acid
Silicotungstic acid or tungstosilicic acid is a heteropoly acid with the chemical formula . It forms hydrates . In freshly prepared samples, ''n'' is approximately 29, but after prolonged desiccation, ''n'' becomes 6. It is a white solid although ...
. A similar process uses the same metal catalyst on silicotungstic acid and silica:
:
It is thought to be competitive with methanol carbonylation for smaller plants (100–250 kt/a), depending on the local price of ethylene.
Oxidative fermentation
For most of human history, acetic acid bacteria of the genus ''Acetobacter
''Acetobacter'' is a genus of acetic acid bacteria. Acetic acid bacteria are characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. Of these, the genus ''Acetobacter'' is distinguished by the ability to oxidiz ...
'' have made acetic acid, in the form of vinegar. Given sufficient oxygen, these bacteria can produce vinegar from a variety of alcoholic foodstuffs. Commonly used feeds include apple cider
Apple cider (also called sweet cider, soft cider, or simply cider) is the name used in the United States and Canada for an unfiltered, unsweetened, non-alcoholic beverage made from apples. Though typically referred to simply as "cider" in North ...
, wine
Wine is an alcoholic drink made from Fermentation in winemaking, fermented fruit. Yeast in winemaking, Yeast consumes the sugar in the fruit and converts it to ethanol and carbon dioxide, releasing heat in the process. Wine is most often made f ...
, and fermented grain
A grain is a small, hard, dry fruit (caryopsis) – with or without an attached husk, hull layer – harvested for human or animal consumption. A grain crop is a grain-producing plant. The two main types of commercial grain crops are cereals and ...
, malt
Malt is any cereal grain that has been made to germinate by soaking in water and then stopped from germinating further by drying with hot air, a process known as "malting".
Malted grain is used to make beer, whisky, malted milk, malt vinegar, ...
, rice
Rice is a cereal grain and in its Domestication, domesticated form is the staple food of over half of the world's population, particularly in Asia and Africa. Rice is the seed of the grass species ''Oryza sativa'' (Asian rice)—or, much l ...
, or potato
The potato () is a starchy tuberous vegetable native to the Americas that is consumed as a staple food in many parts of the world. Potatoes are underground stem tubers of the plant ''Solanum tuberosum'', a perennial in the nightshade famil ...
mashes. The overall chemical reaction facilitated by these bacteria is:
:
A dilute alcohol solution inoculated with ''Acetobacter'' and kept in a warm, airy place will become vinegar over the course of a few months. Industrial vinegar-making methods accelerate this process by improving the supply of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
to the bacteria.
One of the first modern commercial processes was the "fast method" or "German method", first practised in Germany in 1823. In this process, fermentation takes place in a tower packed with wood shavings or charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ca ...
. The alcohol-containing feed is trickled into the top of the tower, and fresh air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
supplied from the bottom by either natural or forced convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoy ...
. The improved air supply in this process cut the time to prepare vinegar from months to weeks.
Nowadays, most vinegar is made in submerged tank culture
Culture ( ) is a concept that encompasses the social behavior, institutions, and Social norm, norms found in human societies, as well as the knowledge, beliefs, arts, laws, Social norm, customs, capabilities, Attitude (psychology), attitudes ...
, first described in 1949 by Otto Hromatka and Heinrich Ebner. In this method, alcohol is fermented to vinegar in a continuously stirred tank, and oxygen is supplied by bubbling air through the solution. Using modern applications of this method, vinegar of 15% acetic acid can be prepared in only 24 hours in batch process, even 20% in 60-hour fed-batch process.
Anaerobic fermentation
Species ofanaerobic bacteria
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenat ...
, including members of the genus ''Clostridium
''Clostridium'' is a genus of anaerobic, Gram-positive bacteria. Species of ''Clostridium'' inhabit soils and the intestinal tracts of animals, including humans. This genus includes several significant human pathogens, including the causative ...
'' or ''Acetobacterium
''Acetobacterium'' is a genus of anaerobic, Gram-positive bacteria that belong to the Eubacteriaceae family.
The type species of this genus is ''Acetobacterium woodii''. The name, ''Acetobacterium'', has originated because they are acetogens, ...
'', can convert sugars to acetic acid directly without creating ethanol as an intermediate. The overall chemical reaction conducted by these bacteria may be represented as:
:
These acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic resp ...
ic bacteria produce acetic acid from one-carbon compounds, including methanol, carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, or a mixture of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
:
:
This ability of ''Clostridium'' to metabolize sugars directly, or to produce acetic acid from less costly inputs, suggests that these bacteria could produce acetic acid more efficiently than ethanol-oxidizers like ''Acetobacter''. However, ''Clostridium'' bacteria are less acid-tolerant than ''Acetobacter''. Even the most acid-tolerant ''Clostridium'' strains can produce vinegar in concentrations of only a few percent, compared to ''Acetobacter'' strains that can produce vinegar in concentrations up to 20%. At present, it remains more cost-effective to produce vinegar using ''Acetobacter'', rather than using ''Clostridium'' and concentrating it. As a result, although acetogenic bacteria have been known since 1940, their industrial use is confined to a few niche applications.
Uses
Acetic acid is a chemicalreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
for the production of chemical compounds. The largest single use of acetic acid is in the production of vinyl acetate monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
, closely followed by acetic anhydride and ester production. The volume of acetic acid used in vinegar is comparatively small.
Vinyl acetate monomer
The primary use of acetic acid is the production ofvinyl acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
Prod ...
monomer (VAM). In 2008, this application was estimated to consume a third of the world's production of acetic acid. The reaction consists of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
and acetic acid with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
over a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, conducted in the gas phase.
:
Vinyl acetate can be polymerized to polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue (a term that may also refer to other types of glues), PVA glue, white glue, carpenter's glue, school glue, or Elmer's Glue in the US, is a widely available adh ...
or other polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
, which are components in paint
Paint is a material or mixture that, when applied to a solid material and allowed to dry, adds a film-like layer. As art, this is used to create an image or images known as a painting. Paint can be made in many colors and types. Most paints are ...
s and adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantage ...
s.
Ester production
The majorester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s of acetic acid are commonly used as solvents for ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. ...
s, paint
Paint is a material or mixture that, when applied to a solid material and allowed to dry, adds a film-like layer. As art, this is used to create an image or images known as a painting. Paint can be made in many colors and types. Most paints are ...
s and coating
A coating is a covering that is applied to the surface of an object, or substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. powder coatings.
Paints ...
s. The esters include ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
, ''n''-butyl acetate
''n''-Butyl acetate is an organic compound with the formula . A colorless, flammable liquid, it is the ester derived from ''n''-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet s ...
, isobutyl acetate
The chemical compound isobutyl acetate, also known as 2-methylpropyl ethanoate (IUPAC name) or β-methylpropyl acetate, is a common solvent. It is produced from the esterification of isobutanol with acetic acid. It is used as a solvent for lacqu ...
, and propyl acetate
Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fra ...
. They are typically produced by catalyzed
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
reaction from acetic acid and the corresponding alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
:
:, R = general alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
For example, acetic acid and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
gives ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
.
:
Most acetate esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
, however, are produced from acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
using the Tishchenko reaction
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde in the presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alk ...
. In addition, ether acetates are used as solvents for nitrocellulose
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and ...
, acrylic lacquers, varnish removers, and wood stains. First, glycol monoethers are produced from ethylene oxide or propylene oxide with alcohol, which are then esterified with acetic acid. The three major products are ethylene glycol monoethyl ether acetate (EEA), ethylene glycol monobutyl ether acetate (EBA), and propylene glycol monomethyl ether acetate (PMA, more commonly known as PGMEA in semiconductor manufacturing processes, where it is used as a resist solvent). This application consumes about 15% to 20% of worldwide acetic acid. Ether acetates, for example EEA, have been shown to be harmful to human reproduction.
Acetic anhydride
The product of the condensation reaction, condensation of two molecules of acetic acid isacetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
. The worldwide production of acetic anhydride is a major application, and uses approximately 25% to 30% of the global production of acetic acid. The main process involves dehydration of acetic acid to give Ethenone, ketene at 700–750 °C. Ketene is thereafter reacted with acetic acid to obtain the anhydride:
:
:
Acetic anhydride is an acetylation agent. As such, its major application is for cellulose acetate
In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and ...
, a synthetic textile also used for photographic film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin photographic emulsion, emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the ...
, which is produced by reacting cellulose with acetic acid and acetic anhydride in the presence of sulfuric acid. Acetic anhydride is also a reagent for the production of heroin and other compounds.
Use as solvent
As a polarprotic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labi ...
, acetic acid is frequently used for recrystallization (chemistry), recrystallization to purify organic compounds. Acetic acid is used as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
in the production of terephthalic acid (TPA), the raw material for polyethylene terephthalate (PET). In 2006, about 20% of acetic acid was used for TPA production.
Acetic acid is often used as a solvent for reactions involving carbocations, such as Friedel-Crafts#Friedel–Crafts alkylation, Friedel-Crafts alkylation. For example, one stage in the commercial manufacture of synthetic camphor involves a Wagner-Meerwein rearrangement of camphene to isobornyl acetate; here acetic acid acts both as a solvent and as a nucleophile to trap the rearrangement reaction, rearranged carbocation.
Glacial acetic acid is used in analytical chemistry for the estimation of weakly alkaline substances such as organic amides. Glacial acetic acid is a much weaker base (chemistry), base than water, so the amide behaves as a strong base in this medium. It then can be titrated using a solution in glacial acetic acid of a very strong acid, such as perchloric acid.
Medical use
Acetic acid injection into a tumor has been used to treat cancer since the 1800s. Acetic acid is used as part of cervical cancer screening in many areas in the developing world. The acid is applied to the cervix and if an area of white appears after about a minute the test is positive. Acetic acid is an effective antiseptic when used as a 1% solution, with broad spectrum of activity against streptococci, staphylococci, pseudomonas, enterococci and others. It may be used to treat skin infections caused by pseudomonas strains resistant to typical antibiotics. While diluted acetic acid is used in iontophoresis, no high quality evidence supports this treatment for rotator cuff disease. As a treatment for otitis externa, it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines.Foods
Acetic acid has per 100 g. Vinegar is typically no less than 4% acetic acid by mass. Legal limits on acetic acid content vary by jurisdiction. Vinegar is used directly as a condiment, and in the pickling of vegetables and other foods. Table vinegar tends to be more diluted (4% to 8% acetic acid), while commercial food pickling employs solutions that are more concentrated. The proportion of acetic acid used worldwide as vinegar is not as large as industrial uses, but it is by far the oldest and best-known application.Reactions
Organic chemistry
Acetic acid undergoes the typical chemical reactions of a carboxylic acid. Upon treatment with a standard base, it converts to metalacetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
. With strong bases (e.g., organolithium reagents), it can be doubly deprotonated to give . Reduction of acetic acid gives ethanol. The OH group is the main site of reaction, as illustrated by the conversion of acetic acid to acetyl chloride. Other substitution derivatives include acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
; this anhydride is produced by Condensation reaction, loss of water from two molecules of acetic acid. Esters of acetic acid can likewise be formed via Fischer esterification, and amides can be formed. When heated above , acetic acid decomposes to produce carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and methane, or to produce ketene and water:
:
:
Reactions with inorganic compounds
Acetic acid is mildly corrosion, corrosive to metals including iron, magnesium, and zinc, forminghydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas and salts called acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
s:
:
Because aluminium forms a Passivation (chemistry), passivating acid-resistant film of aluminium oxide, aluminium tanks are used to transport acetic acid. Containers lined with glass, stainless steel or polyethylene are also used for this purpose. Metal acetates can also be prepared from acetic acid and an appropriate Base (chemistry), base, as in the popular "Sodium bicarbonate, baking soda + vinegar" reaction giving off sodium acetate:
:
A colour reaction for salts of acetic acid is iron(III) chloride solution, which results in a deeply red colour that disappears after acidification. A more sensitive test uses lanthanum nitrate with iodine and ammonia to give a blue solution. Acetates when heated with arsenic trioxide form cacodyl oxide, which can be detected by its odour, malodorous vapours.
Other derivatives
Organic or inorganic salts are produced from acetic acid. Some commercially significant derivatives: *Sodium acetate, used in the textile industry and as a food preservative (E number, E262). *Copper(II) acetate, used as apigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
and a fungicide.
*Aluminium acetate and iron(II) acetate—used as mordants for dyes.
*Palladium(II) acetate, used as a catalyst for organic coupling reactions such as the Heck reaction.
Halogenated acetic acids are produced from acetic acid. Some commercially significant derivatives:
*Chloroacetic acid (monochloroacetic acid, MCA), dichloroacetic acid (considered a by-product), and trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are cal ...
. MCA is used in the manufacture of indigo dye
Indigo dye is an organic compound with a distinctive indigo, blue color. Indigo is a natural dye obtained from the leaves of some plants of the Indigofera#Uses, ''Indigofera'' genus, in particular ''Indigofera tinctoria''. Dye-bearing ''Indigofer ...
.
*Bromoacetic acid, which is esterified to produce the reagent ethyl bromoacetate.
*Trifluoroacetic acid, which is a common reagent in organic synthesis.
Amounts of acetic acid used in these other applications together account for another 5–10% of acetic acid use worldwide.
Health and safety
Vapour
Prolonged inhalation exposure (eight hours) to acetic acid vapours at 10 ppm can produce some irritation of eyes, nose, and throat; at 100 ppm marked lung irritation and possible damage to lungs, eyes, and skin may result. Vapour concentrations of 1,000 ppm cause marked irritation of eyes, nose and upper respiratory tract and cannot be tolerated. These predictions were based on Animal testing, animal experiments and industrial exposure. In 12 workers exposed for two or more years to an airborne average concentration of 51 ppm acetic acid (estimated), symptoms of conjunctive irritation, upper respiratory tract irritation, and hyperkeratotic dermatitis were produced. Exposure to 50 ppm or more is intolerable to most persons and results in intensive Tears, lacrimation and irritation of the eyes, nose, and throat, with pharyngeal oedema and chronic bronchitis. Unacclimatized humans experience extreme eye and nasal irritation at concentrations in excess of 25 ppm, and conjunctivitis from concentrations below 10 ppm has been reported. In a study of five workers exposed for seven to 12 years to concentrations of 80 to 200 ppm at peaks, the principal findings were blackening and hyperkeratosis of the skin of the hands, conjunctivitis (but no corneal damage), bronchitis and pharyngitis, and erosion of the exposed teeth (incisors and canines).Solution
Concentrated acetic acid (≥ 25%) is corrosion, corrosive to skin. These burns or blisters may not appear until hours after exposure. The hazardous properties of acetic acid are dependent on the concentration of the (typically Aqueous solution, aqueous) solution, with the most significant increases in hazard levels at thresholds of 25% and 90% acetic acid concentration by weight. The following table summarizes the hazards of acetic acid solutions by concentration: Concentrated acetic acid can be ignited only with difficulty at standard temperature and pressure, but becomes a flammable risk in temperatures greater than , and can form explosive mixtures with air at higher temperatures with explosive limits of 5.4–16% concentration.See also
*Acetic acid (data page) *Acids in wineNotes
References
External links
*National Pollutant Inventory – Acetic acid fact sheet
(US Permissible exposure limits)
*Calculation o
vapor pressure
of acetic acid
Acetic acid bound to proteins
in the Protein Data Bank, PDB
Swedish Chemicals Agency. Information sheet – Acetic Acid
* Process Flow sheet of Acetic acid Production by th
Carbonylation of Methanol
{{Authority control Acetic acids, Acids in wine Antiseptics Commodity chemicals E-number additives Flavors Household chemicals Otologicals Photographic chemicals Solvents World Health Organization essential medicines Organic compounds with 2 carbon atoms Acetyl compounds