|
Thiocarbamate
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix ''thio-'' suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: ''O''-thiocarbamates, (esters), and ''S''-thiocarbamates, (thioesters). Synthesis Thiocarbamates can be synthesised by the reaction of water or alcohols upon thiocyanates (Riemschneider thiocarbamate synthesis): :RSCN + H2O → RSC(=O)NH2 :RSCN + R'OH → RSC(=O)NR'H Similar reactions are seen between alcohols and thiocarbamoyl chlorides such as dimethylthiocarbamoyl chloride; as well as between thiols and cyanates. The herbicide Cycloate is produced in this way: : Other related thiocarbamate herbicides include vernolate and triallate (. Salts of thiocarbamate arise by the reaction of amines with carbonyl sulfide: : Reactions In the Newman-Kwart rearrangement ''O''-thiocarbamates can isomerise to ''S''-thiocarbamates. This reaction, which generally require ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only one oxygen is replaced the result is thiocarbamate). Dithiocarbamate also refers to the dithiocarbamate ion and its salts. A common example is sodium diethyldithiocarbamate . Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: : Ammonia reacts with carbon disulfide, similarly, to give ammonium dithiocarbamate: : Dithiocarbamate salts are pale colored solids that are soluble in water and Solvent, polar organic solvents. Dithiocarbamic acid A primary amine and carbon disulfide react to give a dithiocarbamic acid: : In the presence of diimides or pyridine, these acids convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-Ethyl Dipropylthiocarbamate
''S''-Ethyl dipropylthiocarbamate (EPTC) is a selective herbicide used for pre-emergent control of certain grasses and broadleaf weeds in Australia and the United States. It was introduced in 1957. Lewis, K.A., Tzilivakis, J., Warner, D. and Green, A. (2016) An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4), 1050-1064. DOI: 10.1080/10807039.2015.1133242 EPTC can be applied pre-emergently or post-emergently and its effectiveness does not depend on post-application rainfall. The herbicide takes effect quickly after application. It is registered in every US state. It should be sprayed when the soil is well worked and dry, to allow good mixing and incorporation. It can be stored at temperatures as low as -50 °F. It is not persistent in soil, having a half-life of about 6 days. Use EPTC is applied at rates of 2 to 7.5 lbs/ac in the US, or 2.5-5 kg/ha in Australia, measured by active ingre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
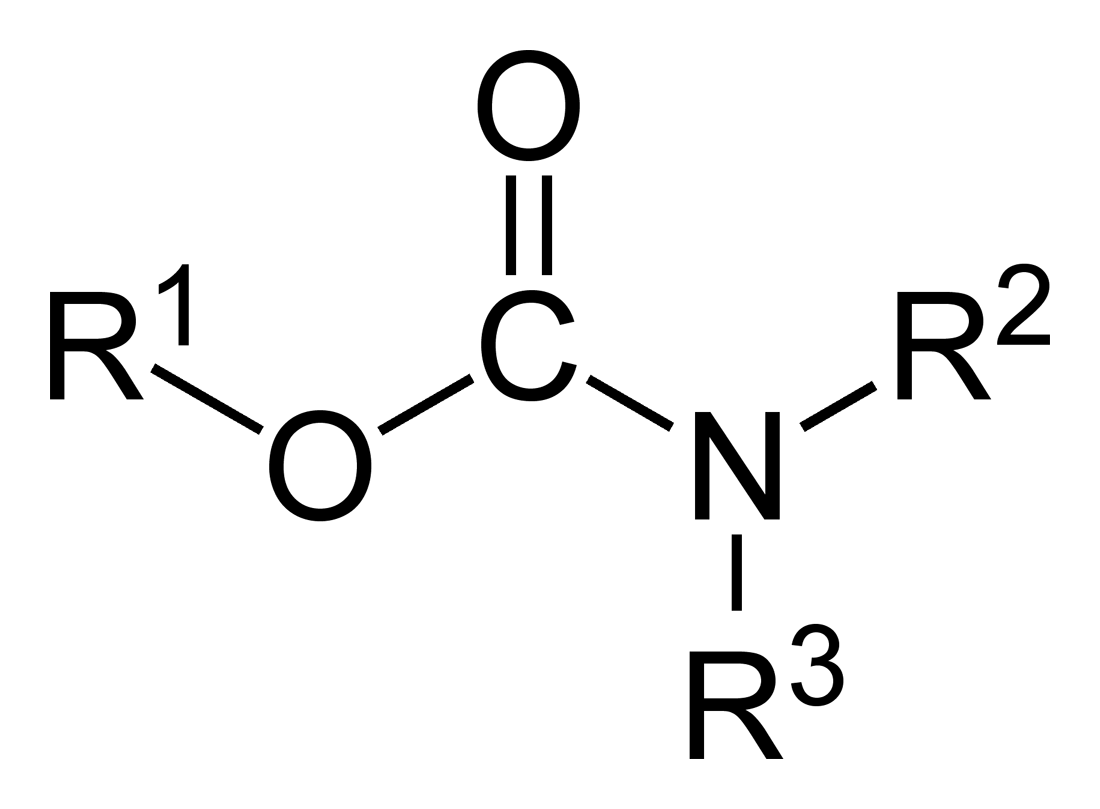
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salt (chemistry), salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose repeat units are joined by carbamate like groups are an important family of plastics, the polyurethanes. See for clarification. Properties While carbamic acids are unstable, many carbamate esters and salt (chemistry), salts are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riemschneider Thiocarbamate Synthesis
The Riemschneider thiocarbamate synthesis converts alkyl or aryl thiocyanates to thiocarbamates under acidic conditions, followed by hydrolysis with ice water. The reaction was discovered by the German chemist in 1951 as a more efficient method to produce thiocarbamates. Some references spell the name ''Riemenschneider''. image:riemschneider.png, 500px, center The Riemschneider reaction can also be used to create the corresponding ''N''-substituted thiocarbamate from an Alcohol (chemistry), alcohol or alkene. Mechanism The mechanism for the conversion of an alcohol to the N-substituted thiocarbamate is shown below. The reaction proceeds under acidic conditions. The alcohol accepts a hydrogen ion from sulfuric acid to form a water, which then leaves, creating a carbocation. The Mesomeric effect, mesomeric form of the Nitrile, cyanogroup reacts with the carbocation. The carbocation is attacked by a water, which then loses a hydrogen to form the product. The product then undergoes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butylate (herbicide)
Butylate or butilate is a widely used thiocarbamate herbicide. As a herbicide, it was introduced in 1962,Lewis, K.A., Tzilivakis, J., Warner, D. and Green, A. (2016) An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4), 1050-1064. DOI: 10.1080/10807039.2015.1133242 and it quickly became the fourth most used herbicide in the US, with used in 1974. Its use has declined significantly, to in 1991 to by 1998. It is used on corn (field, sweet, and popcorn), to control grassy and broadleaf weeds and nutsedge. Application Butylate is applied as an emulsifiable concentrate of 85% active ingredient and is incorporated into the soil, being applied preplant, at plant, postplant, or after harvest. Its maxmimum application rate is 6.3 lb/acre (7.1 kg/Ha), which is much higher than many other herbicides. Soil incorporation is necessary due to the high volatility. Granular and encapsulated forms are als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tolnaftate
Tolnaftate (INN), sold under the brand name TAGRID, among others, is a synthetic thiocarbamate used as an anti-fungal agent that may be sold without medical prescription in most jurisdictions. It is supplied as a cream, powder, spray, liquid, and liquid aerosol. Tolnaftate is used to treat fungal conditions such as jock itch, athlete's foot and ringworm. Tolnaftate was discovered by Teruhisa Noguchi in 1962 while he was working for the Nippon Soda Company. Mechanism Although the exact mechanism of action is not entirely known, it is believed to inhibit squalene epoxidase, an important enzyme in the biosynthetic pathway of ergosterol (a key component of the fungal cell membrane) in a similar way to terbinafine. Uses Tolnaftate has been found to be generally slightly less effective than azoles when used to treat tinea pedis (athlete's foot). It is, however, useful when dealing with ringworm, especially when passed from pets to humans.Crawford F, Hart R, Bell-Syer S, Torgerson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triallate
Tri-allate or triallate is a selective pre-emergent thiocarbamate herbicide, used to control wild oats and sundry grasses and broadleaf weeds, often co-applied with trifluralin, which is much weaker against wild oats than tri-allate. Residual control can be expected for 6 to 8 weeks. It is used in Australia, and the United States. It was first registered in 1961. In 2001, US agriculture used annually. Tri-allate is a mitosis-inhibitor; its HRAC classification is Group J (Aus), Group K3 (global), Group 15 (numeric). Affected weeds are prevented from germinating, or their shoots will be swollen and bright green. Environmental behaviour Under very dry conditions, tri-allate can persist in soil for several months, and can damage field oats and sorghum. Degradation is dependent on soil microörganisms and moisture levels. Tri-allate is nontoxic to birds and bees, though it is very toxic to aquatic life. It doesn't bioaccumulate in plants, and has low mobility in soil. The aquatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Thiocyanates
Organic thiocyanates are organic compounds containing the functional group RSCN. the organic group is attached to sulfur: R−S−C≡N has a S–C single bond and a C≡N triple bond. Organic thiocyanates are valued building blocks. They allow to access efficiently various sulfur containing functional groups and scaffolds. Synthesis Several synthesis routes exist, the most common being the reaction between alkyl halides and alkali thiocyanate in aqueous media. Illustrative is the preparation of isopropyl thiocyanate by treatment of isopropyl bromide with sodium thiocyanate in boiling ethanol. The main complication with this route is the competing formation of alkyisothiocyanates. "SN1-type" substrates (e.g., benzyl halides) tend to give the isothiocyanate derivatives. Some organic thiocyanates are generated by cyanation of some organosulfur compounds. Sulfenyl thiosulfates (RSSO3−) react with alkali metal cyanides to give thiocyanates with displacement of sulfite. This a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylthiocarbamoyl Chloride
Dimethylthiocarbamoyl chloride is an organosulfur compound with the formula (CH3)2NC(S)Cl. A yellow solid, it is often encountered as a yellow syrup. It is a key reagent in the synthesis of arylthiols via the Newman-Kwart rearrangement. Synthesis and reactions Representative of other thiocarbamoyl chlorides, dimethylthiocarbamoyl chloride is electrophilic, serving as a source of R2NC(S)+.R. J. Cremlyn "An Introduction to Organosulfur Chemistry" John Wiley and Sons: Chichester (1996). It is analogous to dimethylcarbamoyl chloride (R2NC(O)Cl). Dimethylthiocarbamoyl chloride is prepared by chlorination of the related tetramethylthiuram disulfide: : e2NC(S)sub>2S2 + 3 Cl2 → 2 Me2NC(S)Cl + 2 SCl2 Dimethylthiocarbamoyl chloride reacts with dithiocarbamates (R2NCS{{su, b=2, p=−) to give thiuram sulfides 2NC(S)sub>2S. With methanethiolate, it gives methyl dimethyldithiocarbamate Methyl dimethyldithiocarbamate is the organosulfur compound with the formula . It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Goitrin
Goitrin is an organosulfur compound classified as a derivative of oxazolidine and as a cyclic thiocarbamate. It reduces the production of thyroid hormones such as thyroxine. It is found in cruciferous vegetables such as cabbage, brussels sprouts and rapeseed oil, and is formed by the hydrolysis of a glucosinolate: progoitrin or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate (2-hydroxy-3-butenyl isothiocyanate) derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group (allowing a five-membered ring to be formed). Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate (or glucosinolates such as glucobrassicin and sinalbin which liberate thiocyanate ion) have goitrogenic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid functio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |