Riemschneider Thiocarbamate Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Riemschneider thiocarbamate synthesis converts alkyl or aryl 
 The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) t ...
s to thiocarbamate
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix ''thio-'' suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: ''O''-thiocarbamates, (ester ...
s under acidic conditions, followed by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
with ice water. The reaction was discovered by the German chemist in 1951 as a more efficient method to produce thiocarbamates. Some references spell the name ''Riemenschneider''.
500px, center
The Riemschneider reaction can also be used to create the corresponding ''N''-substituted thiocarbamate from an alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
or alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
.

Mechanism
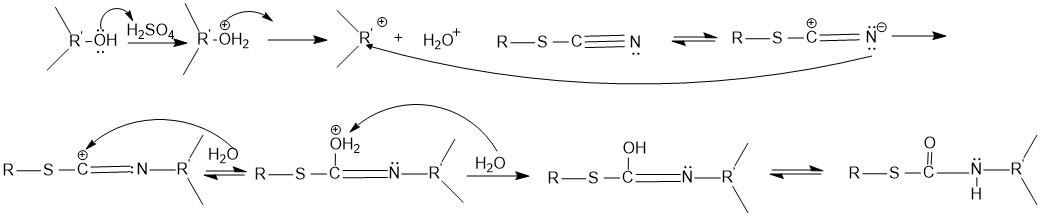
The mechanism for the conversion of an alcohol to the N-substituted thiocarbamate is shown below. The reaction proceeds under acidic conditions. The alcohol accepts a hydrogen ion from sulfuric acid to form a water, which then leaves, creating acarbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
. The mesomeric
In chemistry, the mesomeric effect (or resonance effect) is a property of substituents or functional groups in a chemical compound. It is defined as the Chemical polarity, polarity produced in the molecule by the interaction of two pi bonds or be ...
form of the cyanogroup reacts with the carbocation. The carbocation is attacked by a water, which then loses a hydrogen to form the product. The product then undergoes hydrolysis to form the N-substituted thiocarbamate.
 The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
Uses and Limitations
The Riemschneider thiocarbamate synthesis for aromatic compounds does not work efficiently for ortho-substituted compounds such as ortho-carboxy, ortho-methoxy or ortho-nitro derivative compounds. The reaction is also not as efficient for compounds that are sensitive to concentrated acid, such as thiocyanophenols. The reaction works well for other compounds. Various thiocyanate compounds underwent the Riemschneider synthesis to form thiocarbamates, and all had melting points similar to the predicted value.References